

HTRF Human Phospho-JAK1 (Tyr1034/1035) Detection Kit, 10,000 Assay Points






This HTRF kit enables the cell-based quantitative detection of phosphorylated JAK1 at Tyr1034/1035 as a readout of cytokine receptor activation.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
This HTRF kit enables the cell-based quantitative detection of phosphorylated JAK1 at Tyr1034/1035 as a readout of cytokine receptor activation.









Product information
Overview
JAK1 (Janus kinase 1) belongs to the family of non-receptor Janus tyrosine kinases with JAK2, JAK3, and TYK2. A wide array of cytokines and growth factors ( IL6, IL4, IFNalpha, GM-CSF) attached to their receptors induce the phosphorylation of JAKs. The activated JAKs subsequently phosphorylate additional targets, including both the cytokine receptors and the major substrates: STATs. The JAKs/SATs signaling stimulates cell proliferation, differentiation, migration, and apoptosis. Altering JAK/Stat signaling to reduce cytokine induced pro-inflammatory responses represents an attractive target for anti-inflammatory therapies.
How it works
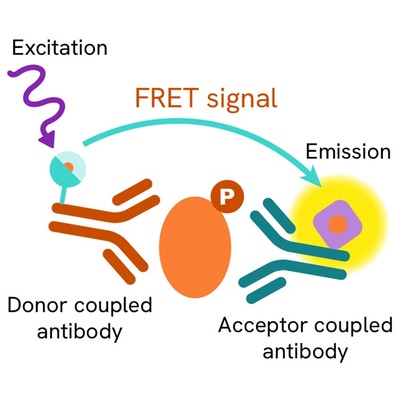
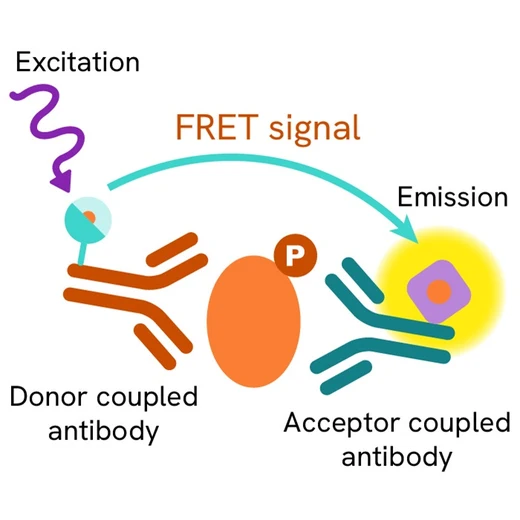
Phospho-JAK1 (Tyr1034/1035) assay principle
The Phospho-JAK1 (Tyr1034/1035) assay measures JAK1 when phosphorylated at Tyr1034/1035. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The Phospho-JAK1 (Tyr1034/1035) assay uses 2 labeled antibodies: one with a donor fluorophore, the other with an acceptor. The first antibody was selected for its specific binding to the phosphorylated motif on the protein, the second for its ability to recognize the protein independent of its phosphorylation state. Protein phosphorylation enables an immune-complex formation involving both labeled antibodies and which brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample, and provides a means of assessing the protein’s phosphorylation state under a no-wash assay format.

Phospho-JAK1 (Tyr1034/1035) 2-plate assay protocol
The 2 plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before adding Phospho-JAK1 (Tyr1034/1035) HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Phospho-JAK1(Tyr1034/1035) 1-plate assay protocol
Detection of Phosphorylated JAK1 (Tyr1034/1035) with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
Inhibition measured with Phospho-JAK1 (Tyr1034/1035) & Total-JAK1 kits on HEL92.1.7 cells
HEL92.1.7 cells (Human erythroleukaemia) were seeded in a half area 96-well culture-treated plate at 300,000 cells / well in 20 µL complete culture medium. Cells were treated with 5 µL of increasing concentrations of Ruxolitinib or Upadacitinib for 1h at 37°C, 5% CO₂ followed by a stimulation step with 5 µL of pervanadate 100 µM, IL4 100 ng/mL and IFNg 100 ng/mL mixture during 30 minutes . After treatment, cells were lysed with 10µl of supplemented lysis buffer # 4 (4X) for 30 min at RT under gentle shaking.
After cell lysis, 16 µL of lysate were transferred into a 384-well sv white microplate, and 4 µL of the HTRF phospho-JAK1 (Tyr1034/1035) or Total JAK1 detection reagents were added. The HTRF signal was recorded after an overnight incubation at room temperature.
As expected, the results obtained show a dose-response inhibition of JAK1 Y1034/1035 phosphorylation upon treatment with Ruxolitinib or Upadacitinib, while the JAK1 expression level remains constant.


Inhibition of JAK1 phosphorylation (Tyr1034/1035) on Hep-G2 cells
Human HEP-G2 cells (hepatocellular carcinoma) were plated in 96-well culture-treated plate (400,000 cells/well) in complete culture medium, and incubated 6H at 37°C,5% CO₂. Cells were treated with a dose-response of Ruxolitinib or Upadacitinib overnight at 37 °C, 5% CO₂. Cells were stimulated with pervanadate 100 µM, IL6 100 ng/mL, IL4 100 ng/mL and IFNg 100 ng/mL during 1 hour at 37°C,5% CO₂. Cells were then lysed with 25 µl of supplemented lysis buffer #4 (1X) for 30 min at RT under gentle shaking. After cell lysis, 16 µL of lysate were transferred into a 384-well sv white microplate and 4 µL of the HTRF Phospho-JAK1 (Tyr1034) or Total-JAK1 detection reagents were added. The HTRF signal was recorded after an overnight incubation at room temperature.
As expected, both inhibitors induced a dose-dependent decrease in JAK1 phosphorylation, without effect on the expression level of the total protein.


Phospho JAK1 (Tyr1034/1035) down-regulation by siRNA
HEL92.1.7 cells were treated with 2.5 µM of Accell siRNA (Horizon) targeting specifically JAK1, JAK2, JAK3 or with a non-targeting siRNA (included as control), in a 96-well plate (200,000 cells/well) under 100 µL. After 48h incubation at 37°C, 50 µL of complete culture medium was added and the cells were incubated for an additional 24h-incubation at 37°C. Cells were stimulated with Pervanadate (100 µM) IL4 and IFN gamma during 30 minutes and after plate centrifugation, were lysed with 50 µL of supplemented lysis buffer #4 (1X) and 16 µL of lysates were transferred into a low volume white microplate before the addition of 4 µL of premixed HTRF phospho-JAK1(Tyr1034/1035) detection antibodies. The HTRF signal was recorded after an overnight incubation at RT.
Cell treatment with JAK1 siRNA led to a significant downregulation of phospho JAK1 (Tyr1034/1035) with 65% signal decrease compared to the cells transfected with the non-targeting siRNA. No decrease of signal was observed with cells treated with JAK2 or JAK3 siRNA , demonstrating the specificity of the kit.

HTRF Phospho-JAK1(Tyr1034/1035) assay compared to Western Blot
HEL92.1.7 cells were cultured in a T175 flask in complete culture medium at 37°C, 5% CO₂. After 72h incubation, the cells were first stimulated with pervanadate 100 µM, IL4 and IFNg 100 ng/mL, then lysed with 3 mL of supplemented lysis buffer #4 (1X) for 30 minutes at RT under gentle shaking.
Serial dilutions of the cell lysate were performed using supplemented lysis buffer, and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of HTRF Phospho-JAK1 (Tyr1034/1035) detection reagents. Equal amounts of lysates were used for a side by side comparison between HTRF and Western Blot.
A side by side comparison of Western Blot and HTRF demonstrates that the HTRF assay is 4-fold more sensitive than the Western Blot, at least under these experimental conditions.

Simplified pathway
Simplified JAK1 signaling pathway
JAK1 in combination with JAK2, JAK3, or Tyk2 interacts with different type of cytokine/interferon receptors (IL2, IL4, IL6 , IFN alpha and gamma). Upon cytokine activation, JAK1 and the other members of the JAK family transphosphorylate each other, and then activate the transcription factors STAT1, STAT2, STAT3, STAT5, and STAT6 that dimerize. They then translocate to the nucleus to trigger the transcription of genes regulating cell differentiation, proliferation, survival, and adapted immune response.

Specifications
| Application |
Cell Signaling
|
|---|---|
| Automation Compatible |
Yes
|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 2
Lysis Buffer 3
Lysis Buffer 4
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Inflammation
|
| Unit Size |
10,000 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
This technical note explores how pervanadate enhances tyrosine phosphorylation detection, enabling clearer insights into kinase...
This guide provides you an overview of HTRF applications in several therapeutic areas.


Loading...
How can we help you?
We are here to answer your questions.






























