Explore our solutions
Lower the cost of goods for cell therapies
LentiBOOST™ transduction enhancer allows you to decrease the vector quantity required so that you can reduce the cost of goods for cell therapies. Researchers from University of Manchester and UCL have shown an almost 5-fold reduction of vector amounts while achieving equivalent efficacy in a GMP stem cell manufacturing protocol for HSCs (Ellison et al. 2024).
Helping to enhance therapeutic response rates
Improving lentiviral transduction efficiency with LentiBOOST technology can be a way to help enhance therapeutic response rates. For example, in autologous applications when working with exhausted T cells or when there are only a few viable T cells available effective lentiviral transduction can be the difference between a response and no response. This can also be the case when using large constructs.
Improved lentivirus transduction and optimal copy number per cell
Using the LentiBOOST lentivirus transduction enhancer reagent, customers have demonstrated improved transduction without impacting cell health across a range of cell types, including primary T-cells or HSCs which are known to be challenging.
In this example, the number of GFP-positive human CD 34+ PBSC transduced with lentivirus and LentiBOOST technology at various concentrations reached up to 80% at day 12 post transduction.
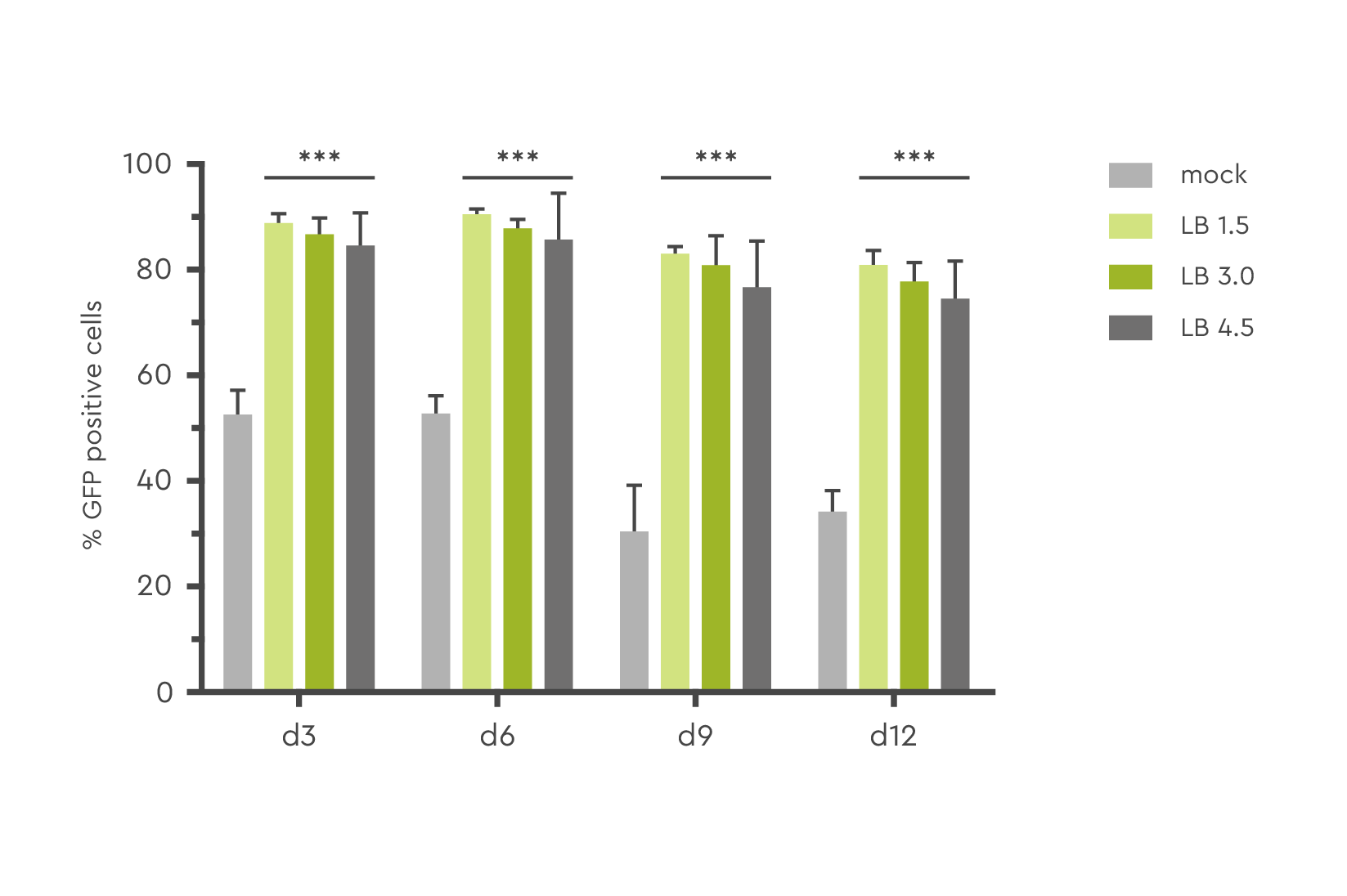
Figure 1: The amount of GFP positive human CD34+ PBSC was determined at day 3, 6, 9, and 12 post transduction at various concentrations of transduction enhancer (LB; 1.5 mg/ml to 4.5 mg/ml). Source: Hauber et al., 2018
LentiBOOST technology allows for adjustable and controllable vector copy numbers per cell allowing for optimization in accordance with EMA/FDA safety guidelines. This helps to facilitate the expression of your therapeutic protein when using various concentrations.
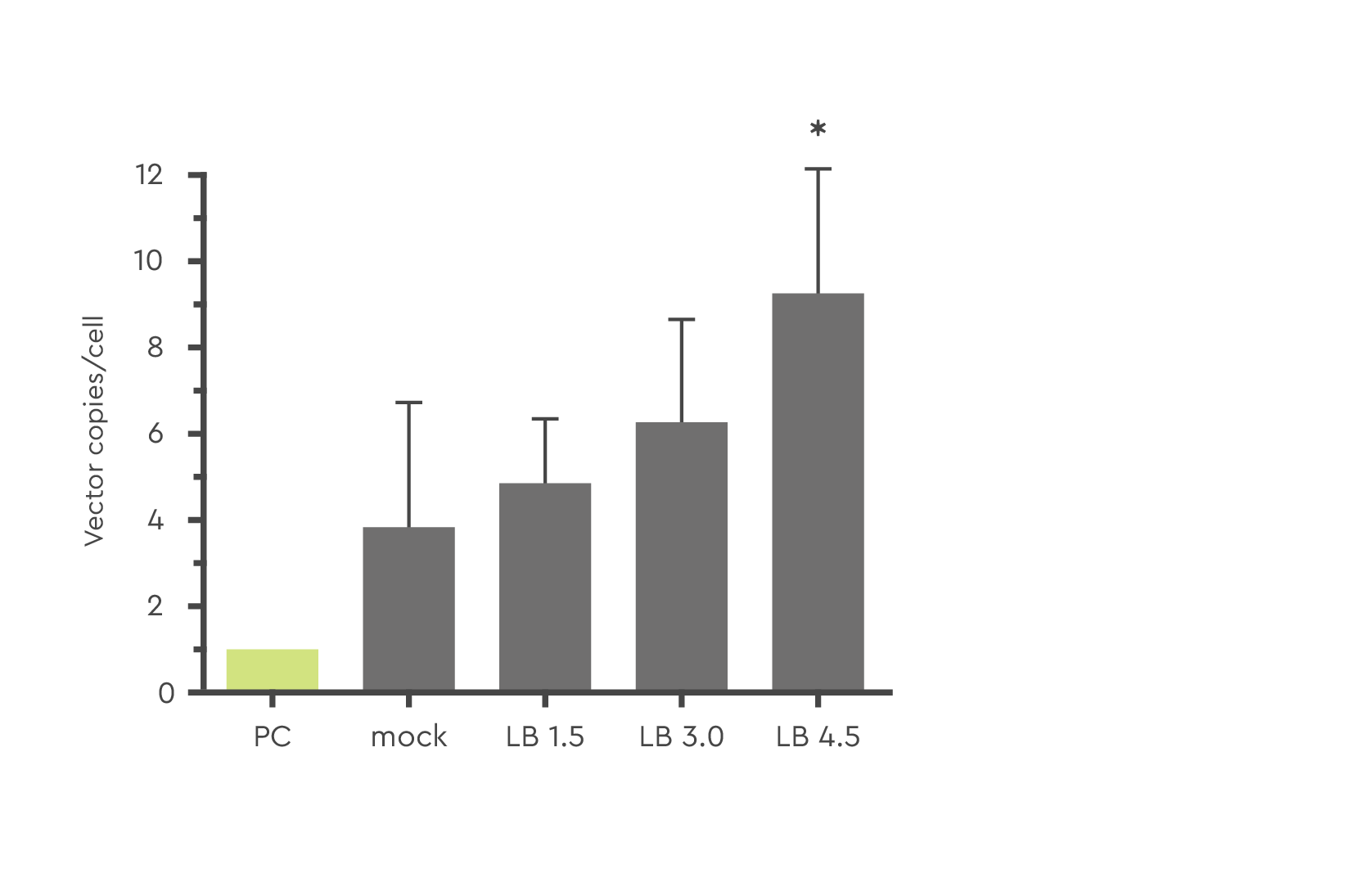
Figure 2: Effect of varying concentrations of LentiBOOST enhancer reagent (1.5 to 4.5 mg/ml) on lentivirus transduction efficiency with LV-GFP (MOI=10) in human CD34+ PBSC. The star (*) signifies p ≤ 0.05. Source: Hauber et al., 2018
A customer’s head-to-head comparison of LentiBOOST technology with other lentivirus transduction enhancers showed that the LentiBOOST platform had the strongest effect on the transduction of the human T cells across the three concentrations they tested. At the highest multiplicity of infection (MOI), the transduction enhancing effect of the LentiBOOST platform was the strongest, with efficiency about 5-times more than lentivirus transduction without an enhancer.
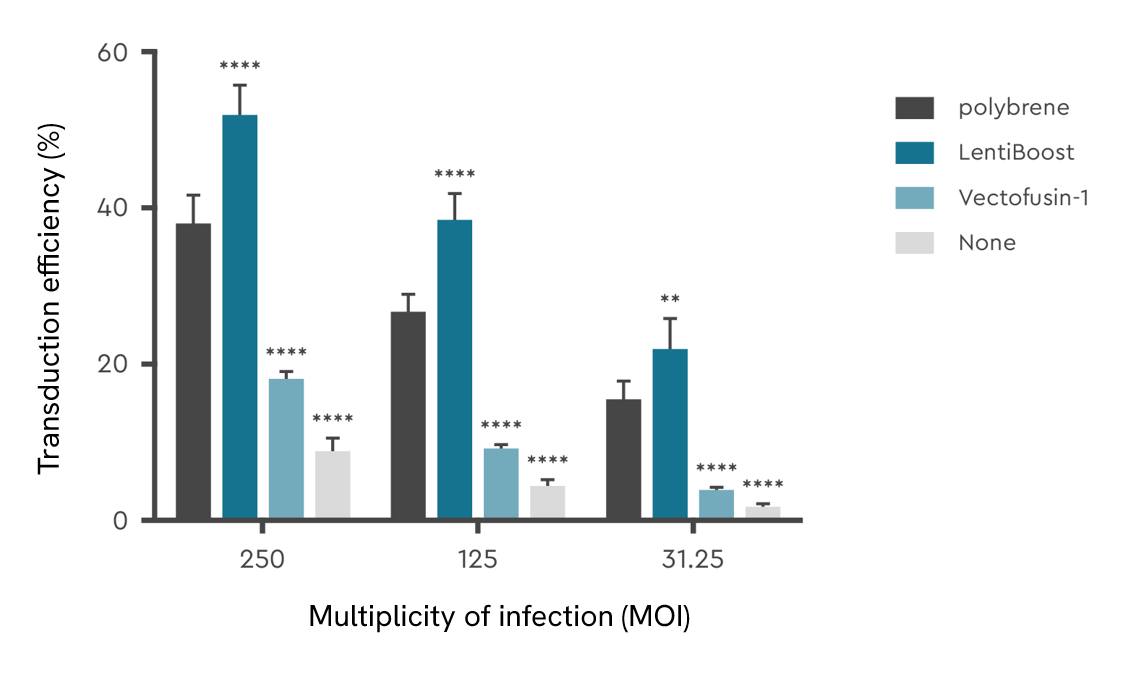
Effective in a wide range of cell types
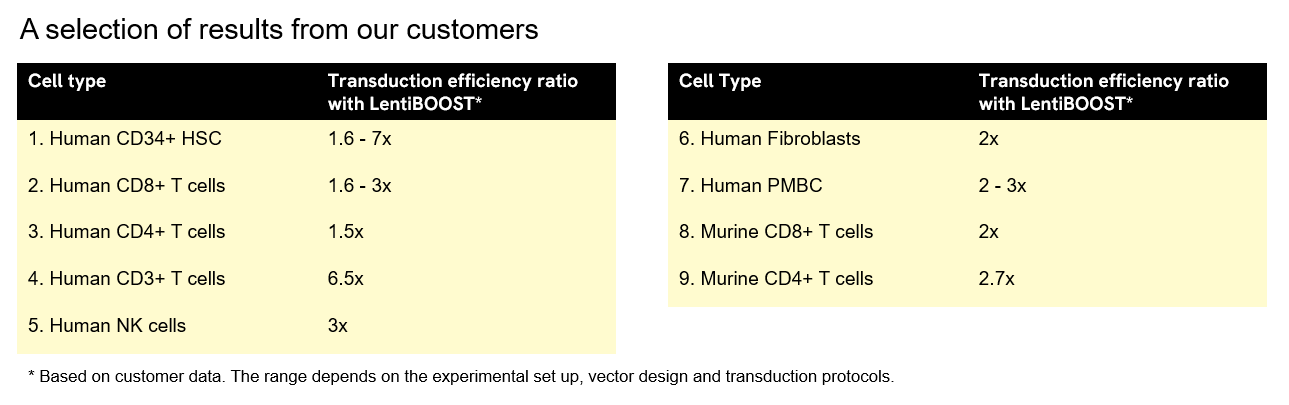
LentiBOOST lentivirus transduction enhancer can be applied to a wide range of clinically relevant cell types, including CD34+ hematopoietic stem cells (HSC), mesenchymal stem cells (MSC), neuronal stem cells, primary T cells, hard-to-transduce murine T cells, NK cells, and fibroblasts. The technology is ideal for clinical transduction protocols for ex vivo gene therapies and CAR-T cell therapies.
Below we show the unit-fold increase in transduced cells when using LentiBOOST enhancer based on customer data. The range depends on the experimental setup, vector design, and transduction protocols.
No cytotoxicity observed and differentiation potential maintained
Optimizing lentiviral transduction efficiency must not be done at the expense of cell health or functionality. This has been assessed in various studies and HSC treated with LentiBOOST technology demonstrated the same viability as the control cells.
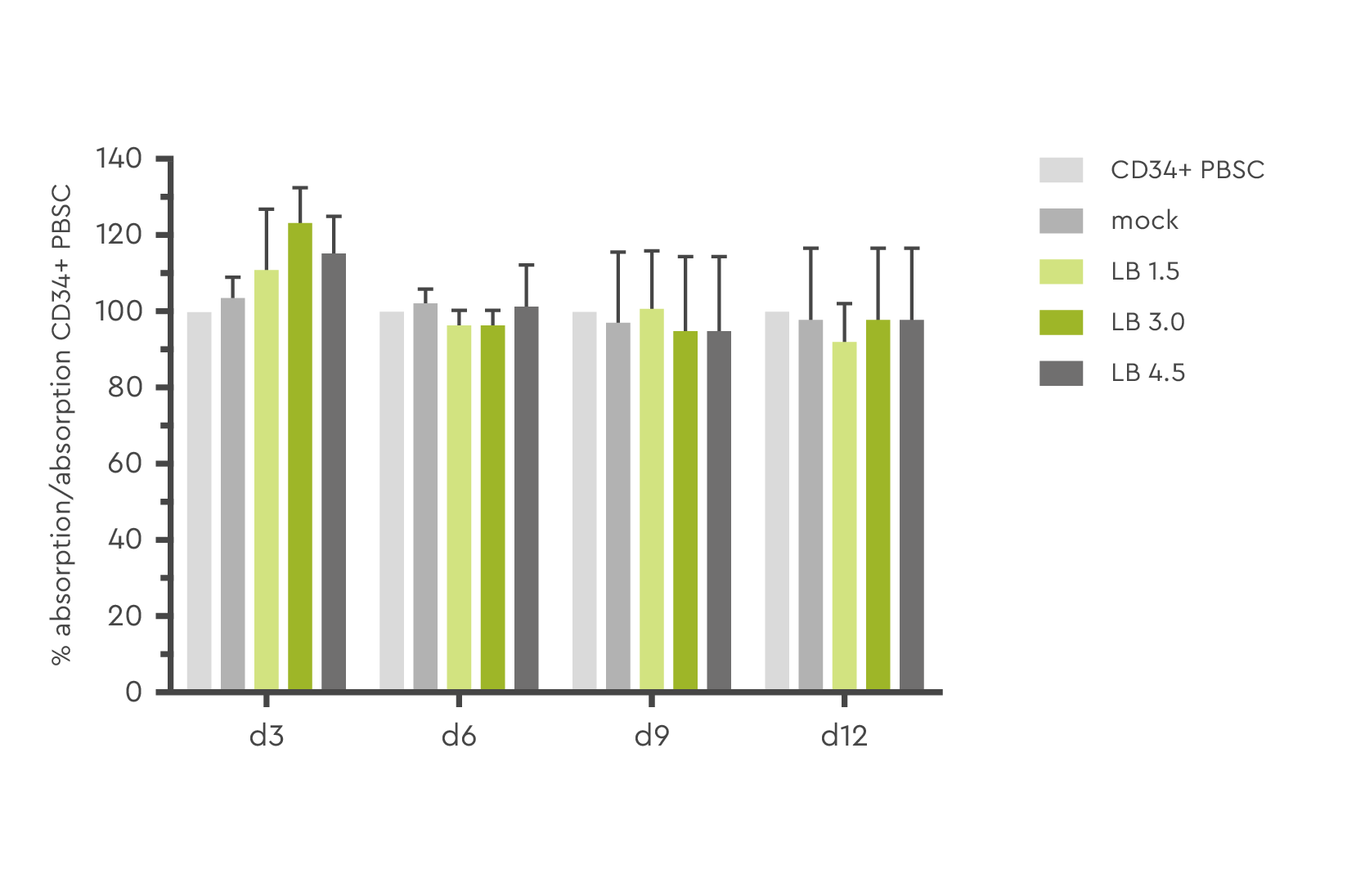
Figure 3: Determination of cell viability and toxicity in a MTT assay at day 3, 6, 9, and 12 post transduction. Source: Hauber et al., 2018
At least as important as health is the differentiation potential of the cells. Experiments have shown that LentiBOOST technology does not affect the ability of HSC to differentiate into various hematopoietic lineages (Hauber et al. 2018).
Successful track record
LentiBOOST™ technology has been integrated into more than 40 Phase I/II and III clinical trials, is used in 2 approved cell and gene therapy products, and has been used by over 600 academic and industry customers worldwide (as of May 2024).
Here is a selection of our LentiBOOST platform users:
- Mustang Bio (NCT01306019): Their MB-207 program aims to assess a lentiviral therapy for the treatment of patients with X-linked severe combined immunodeficiency. The clinical development is a Phase 1/2 study (as of end 2021). More here
- University of California San Francisco (NCT03538899): The goal of this study is to treat patients with Artemis-deficient Severe Combined Immunodeficiency. Patients receive their own transduced stem cells to restore the function of their immune system by introducing a normal copy of the DCLRE1C gene. This treatment is assessed in a Phase 1/2 study (as of end of 2021). More here
- National Institute of Allergy and Infectious Diseases: Their program aims to treat patients with X-linked severe combined immunodeficiency (SCID-X1). Patient CD34+ cells are transduced to express a healthy version of the gene ILR2G that is impaired in patients suffering from SCID-X1. More here
- Orchard Therapeutics: The biopharmaceutical company licensed the LentiBOOST technology to include in its gene therapy products to treat patients with rare diseases. More here
- Cellectis: The biopharmaceutical company licensed LentiBOOST technology to expand its portfolio of technologies for manufacturing its allogeneic CAR-T cells. More here
- Beam Therapeutics: The US company licensed LentiBOOST platform for use in their new generation of CAR-T product candidates using proprietary base editing technology. More here
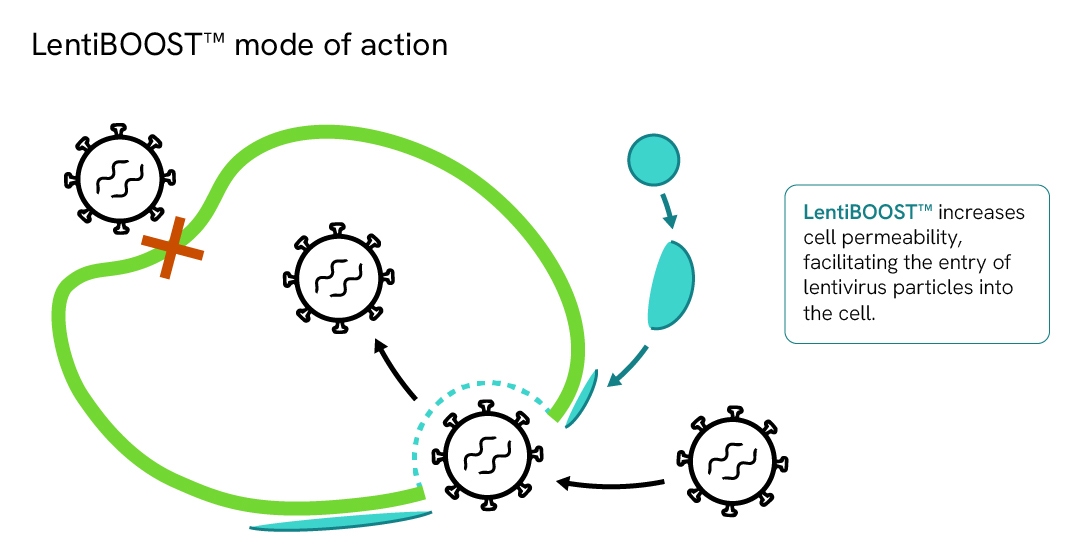
How does LentiBOOST enhancer work?
LentiBOOST technology is a highly effective, non-cytotoxic transduction enhancer for research and clinical application of lentiviral vectors in a wide range of cell types.
It acts as a universal receptor-independent manufacturing aid which facilitates fusion of lentivirus with the cell membrane, increased vector copy number, and significantly improved lentivirus transduction efficiency at lower MOI.
LentiBOOST pharma grade reagent for research use
The LentiBOOST platform is available globally in pharma grade for research and process development purposes. Revvity supplies LentiBOOST pharma grade (non-GMP) material for research and process development including large-scale runs. LentiBOOST pharma grade technology is used in hundreds of laboratories worldwide.
LentiBOOST GMP grade reagent for clinical use
LentiBOOST technology GMP grade material is available for clinical development and for cell therapies at the commercial stage under a clinical or commercial license.
LentiBOOST transduction enhancer helps to increase the response rate and overall success of clinical trials. By integrating the LentiBOOST platform into their processes, our licensees can make their manufacturing more robust and have access to GMP material for both clinical and commercial use.
We know how important a reliable and secure supply chain is, especially in the commercial phase. That’s why we use an automated fill and finish process under GMP conditions in accordance with regulatory requirements. Our quality assurance process ensures compliance with highest standards and batch-to-batch consistency. LentiBOOST technology GMP grade comes with a CoA and all necessary documentation for regulatory authorities such as the FDA and EMA.
We work closely with our licensees as a partner on the road to clinical approval of their cell therapy. To help advance your clinical success, we offer individualized licensing models aligned to help meet your needs.
Revvity offers two main types of licenses:
- Commercial licenses for companies that are developing and commercializing cell therapy programs.
- Academic licenses for investigator-led clinical trials: To support pioneers developing new cell therapies, Revvity offers an academic license for the LentiBOOST technology to academic partners to use in their clinical programs, providing that the non-profit research organization is responsible to the regulatory authorities for the clinical trial.
How much LentiBOOST enhancer do I need?
Material is supplied at a concentration of 100mg/ml. For most applications, LentiBOOST lentivirus transduction enhancer reagent is applied at a dilution of 1:100 to 1:400.