

HTRF Human and Mouse Phospho-STAT1 (Tyr701) Detection Kit, 500 Assay Points






The HTRF Human & Mouse Phospho-STAT1 (Tyr701) Detection Kit is an immunoassay designed for the detection and quantitation of Human & Mouse STAT1 when phosphorylated at Tyr701 in cellular lysates, in a homogeneous (no-wash steps, no separation steps) format.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
The HTRF Human & Mouse Phospho-STAT1 (Tyr701) Detection Kit is an immunoassay designed for the detection and quantitation of Human & Mouse STAT1 when phosphorylated at Tyr701 in cellular lysates, in a homogeneous (no-wash steps, no separation steps) format.









Product information
Overview
This HTRF cell-based assay enables the rapid and quantitative detection of STAT1 phosphorylated on Tyr701 as a readout of JAK/STAT signaling activity. STAT1 acts as an important transcriptional activator and the assay serves as a readout for JAK inhibitors in oncology, virology and inflammation.
HTRF assays offer many advantages over other technologies:
- Homogeneous add-and-read format
- No wash steps
- Low background
- Straightforward miniaturization from 96- or 384-well microplates to high density assay formats such as 384-well low volume and 1536-well plates
- Stable signal, providing flexibility in time of readout or size of assays
How it works
Phospho-STAT1 (Tyr701) assay principle
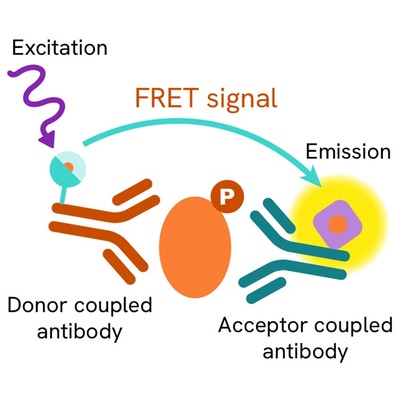
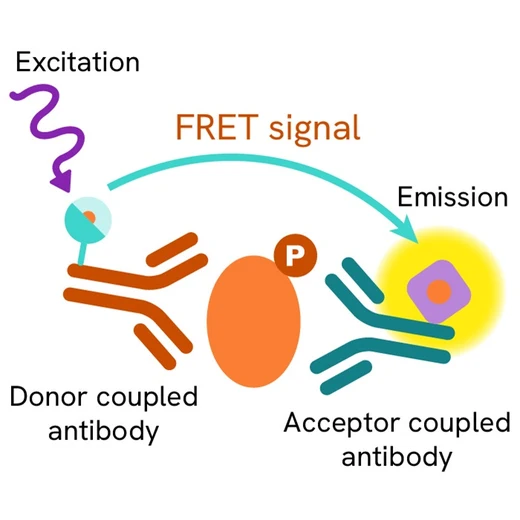
The Phospho-STAT1 (Tyr701) assay measures STAT1 when phosphorylated at Tyr701. Contrary to Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis or transfer. The Phospho-STAT1 (Tyr701) assay uses 2 labeled antibodies: one with a donor fluorophore, the other one with an acceptor. The first antibody is selected for its specific binding to the phosphorylated motif on the protein, the second for its ability to recognize the protein independent of its phosphorylation state. Protein phosphorylation enables an immune-complex formation involving both labeled antibodies and which brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample, and provides a means of assessing the proteins phosphorylation state under a no-wash assay format.

Phospho-STAT1 (Tyr701) 2-plate assay protocol
The 2 plate protocol involves culturing cells in a 96-well plate before lysis then transferring lysates to a 384-well low volume detection plate before adding phospho-STAT1 (Tyr701) HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Phospho-STAT1 (Tyr701) 1-plate assay protocol
Detection of Phosphorylated STAT1 (Tyr701) with HTRF reagents can be performed in a single plate used for culturing, stimulation and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
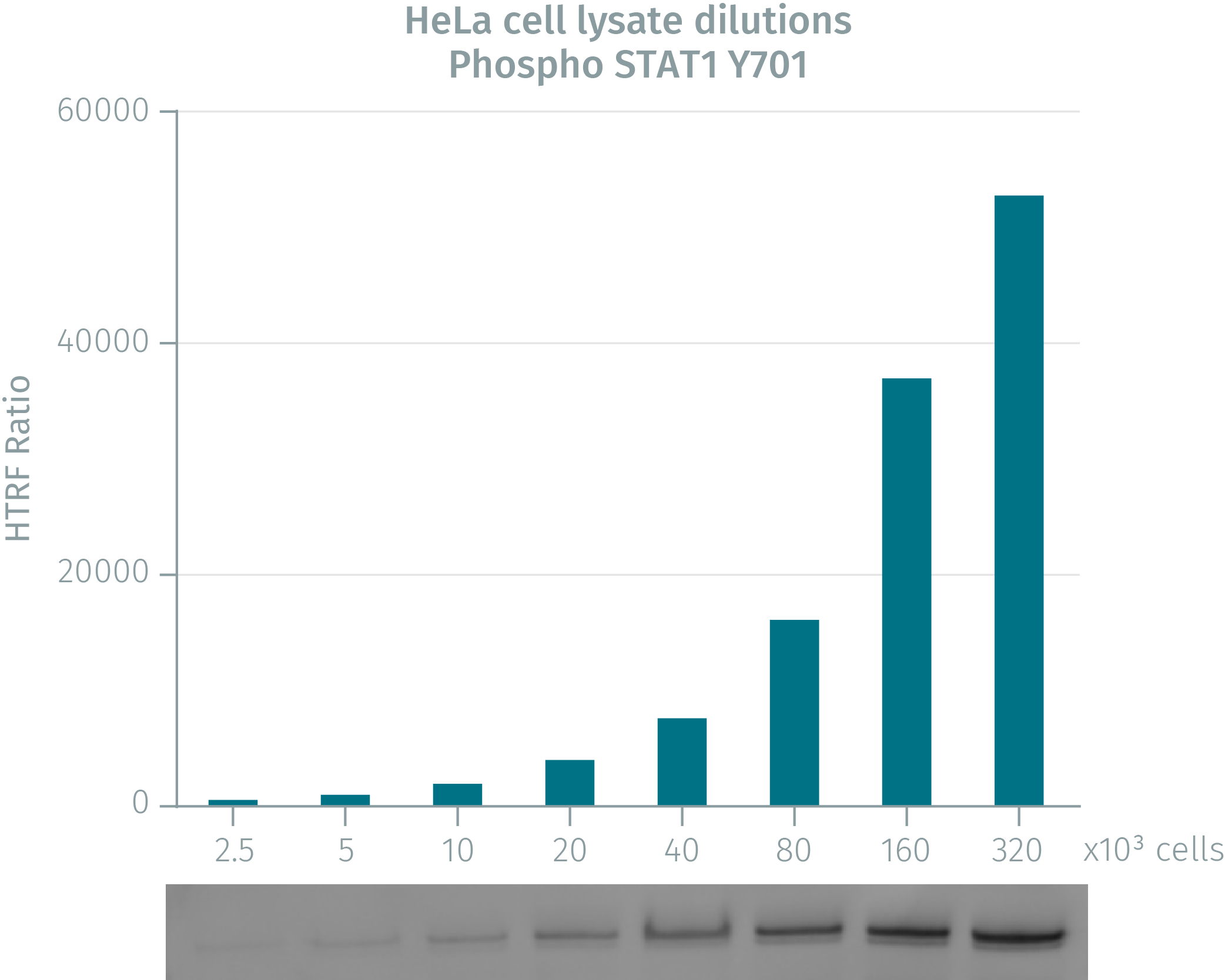
HTRF assay compared to western blot using phospho-STAT1 cellular assays on human HeLa cells
HeLa cells were grown in a T175 flask at 37 °C, 5% CO2 for 48h. Cells were then stimulated with IFNa for 20 min. After medium removal, the cells were lysed with 3mL of supplemented lysis buffer for 30 min at room temperature. Soluble fractions were then collected after 10 min centrifugation. Serial dilutions of the cell lysate were performed in the supplemented lysis buffer and 16 µL of each dilution were dispensed and analyzed side-by-side by Western-blot and by HTRF. For each cell density tested, the fluorescence ratio closely matched the western blot band intensity, demonstrating the reliability of HTRF as an analytical technique for the measurement of phopsho STAT1
Validation of phospho STAT1-Y701 on human & murine cell lines
IFNa was used to induce phosphorylation of STAT1 in mouse NIH 3T3 and human HeLa cells. NIH-3T3 & Hela cells plated at different cell densities, were stimulated with 2.5µg/mL of IFNa for 20 minutes. After a lysis step using 50 µL of supplemented lysis buffer, 16 µL of lysate were transferred into a 384-well low volume white microplate before the addition of 4 µL of the HTRF phospho-STAT1 detection reagents. The HTRF signal was recorded after an overnight incubation. The graph display the biological assay window corresponding to IFNa-stimulated over unstimulated conditions.

Detection of phospho STAT1 on HeLa cells: stimulation kinetic
IFNa was used to induce phosphorylation of STAT1 in HeLa cells. 200,000 cells were plated in a 96well plate, then stimulated with increasing IFNa concentrations for various times (10, 20 30 and 60 minutes). After cell lysis with 50 µL of supplemented lysis buffer, 16 µL of lysate were transferred into a 384-well low volume white microplate before the addition of 4 µL of the HTRF phospho-STAT1 detection reagents. The HTRF signal was recorded after an overnight incubation. In these experimental conditions the optimal stimulation time was determined at 20 minutes of IFNa.

HTRF total-STAT1 assay used to check the phosphorylation status of STAT1 on murine and human cells
After 20 minutes stimulation with increasing IFNa concentrations, NIH 3T3 & Hela cells (200,000 cells/96well plate) were lysed with 50 µL of supplemented lysis buffer and incubated for 30 min at RT under gentle shaking. For phospho- STAT1 detection (blue curve), 16 µL of lysate were transferred into a 384-well low volume white microplate, followed by 4 µL of the HTRF phospho-STAT1 detection reagents For total STAT1 detection (red curve), 16µl of lysate were transferred into a 384-well low volume white, followed by 4 µL of the HTRF Total-STAT1 detection reagents. HTRF signals were recorded after an overnight incubation. Note that the HeLa cells display better potency of IFNa compared to NIH3T3 cells.


Simplified pathway
Transcriptional activator STAT1 involved in the JAK/STAT pathway
STAT1 is an important transcriptional activator involved in the JAK/STAT pathway which is activated by interferon I class, growth factors or chemokines. After stimulation, phosphorylated STAT1 dimers bind to Interferon Stimulated Gene Factor 3 complex. Then STAT1 proteins translocate into the nucleus and activate the transcription of genes associated to cell survival, viability or pathogen response. In response to IFNγ, STAT1 forms homodimers or heterodimers with STAT3 that bind to the GAS (Interferon-Gamma-Activated Sequence) promoter element. In response to either IFNα or IFNß, STAT1 forms heterodimer with STAT2 that bind the Interferon-Stimulated Response Element (ISRE).

Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Infectious Diseases
Neuroscience
Oncology & Inflammation
|
| Unit Size |
500 assay points
|
Video gallery
Citations
Resources
Are you looking for resources, click on the resource type to explore further.
Atherosclerosis pathogenesis, cellular actors, and pathways
Atherosclerosis is a common condition in which arteries harden and...
A comprehensive overview of fibrosis development
Fibrosis is a main contributor to a wide range of organ failures which stems from...
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.
Dive deeper into astrocyte cell research
The release of pro-inflammatory factors by activated astrocytes has been shown to be...
An in-depth review of molecular and cellular pathways
The maintenance of proteostasis, the biological mechanisms that control the...


Loading...
How can we help you?
We are here to answer your questions.






























