Residual DNA detection assays
Our bioprocess quality control solutions are designed to streamline your host cell residual DNA detection workflow, from sample extraction to qPCR quantification. Helping you achieve the purity of your biotherapeutic and CGT products with assays that can demonstrate whether contaminant levels are in compliance with critical quality attributes.

HostDetect CHO PCR DNA Quant Kit
The HostDetect CHO PCR DNA Quant Kit utilizes sequence-specific primers and TaqMan® probe to amplify the Alu-equivalent gene of CHO genomic DNA for residual host genomic identification. A primer/probe set to detect Internal Control (IC), is also included for monitoring entire process from extraction to real-time PCR. The reagents also use a dUTP/UNG carryover prevention system to avoid contamination of PCR products.
This kit is specific for DNA from the CHO genome and cannot detect human or environmental DNA that might be introduced during sample handling.
Key Highlights:
- Quantify CHO genomic DNA with as little as 0.01 pg per PCR reaction in < 2 hours of PCR
- Internal control detection for process monitoring from sample extraction to PCR
- High specificity: No cross-reactivity with unrelated DNA
- Flexible extracted DNA input volume up to 10 µL per well
- Scalable reaction numbers from 1 to up to 192 per kit

HostDetect HEK293 PCR DNA Quant Kit
The HostDetect HEK293 PCR DNA Quant Kit utilizes sequence-specific primers and TaqMan® probe to amplify the Alu-equivalent gene of HEK293 genomic DNA for residual host DNA identification. A primer/probe set to detect Internal Control (IC), is also included for monitoring entire process from extraction to real-time PCR. The reagents also use a dUTP/UNG carryover prevention system to avoid contamination of PCR products.
This kit is specific for DNA from the HEK293 genome and cannot detect human or environmental DNA that might be introduced during sample handling.
For more information, contact: BioprocessQC@revvity.com
Key highlights:
- Quantify HEK293 genomic DNA with as little as 0.03 pg per PCR reaction in < 2 hours of PCR
- Internal control detection for process monitoring from sample extraction to PCR
- High specificity: No cross-reactivity with unrelated DNA
- Flexible extracted DNA input volume up to 10 µL per well
- Scalable reaction numbers from 1 to up to 192 per kit

HostDetect E.coli PCR DNA Quant Kit
The HostDetect E. coli PCR DNA Quant Kit utilizes sequence-specific primers and TaqMan® probe to amplify the 16S ribosomal RNA gene of the E. coli genome for residual host DNA identification. A primer/probe set to detect Internal Control (IC), is also included for monitoring entire process from extraction to real-time PCR. The reagents also use a dUTP/UNG carryover prevention system to avoid contamination of PCR products.
This kit is specific for DNA from the E. coli genome and cannot detect human or environmental DNA that might be introduced during sample handling.
For more information, contact: BioprocessQC@revvity.com
Key highlights:
- Quantify E.coli genomic DNA with as little as 0.03 pg per PCR reaction in < 2 hours of PCR
- Internal control detection for process monitoring from sample extraction to PCR
- High specificity: No cross-reactivity with unrelated DNA
- Flexible extracted DNA input volume up to 10 µL per well
- Scalable reaction numbers from 1 to up to 192 per kit
Host cell protein detection assays
During biotherapeutic manufacturing and production, CHO, HEK 293 and E.coli host cells produce HCP impurities which, if not removed, can induce immunogenicity in individuals or reduce the potency, stability, or effectiveness of a drug. Therefore, to meet regulatory organizations’ guidelines we have developed a range of HCP detection assays to improve your workflows.

HTRF CHO HCP Detection Kit, 500 Assay Points
The CHO Host Cell Protein (HCP) kit measures contaminants originating from the CHO cells used to manufacture therapeutic antibodies. The kit can be used for the quantification of CHO HCP proteins in routine bioprocess operations, from the completely raw harvest material to the final product. The simple and robust procedure benefits from increased throughput compared to ELISA.
HTRF assays offer many advantages over other technologies:
- Homogeneous add-and-read format
- No wash steps
- Low background
- Straightforward miniaturization from 96- or 384-well microplates to high density assay formats such as 384-well low volume and 1536-well plates
- Stable signal, providing flexibility in time of readout or size of assays
AlphaLISA HEK 293 HCP Detection Kit, 100 Assay Points
Human Embryonic Kidney (HEK) 293 is a cell line commonly used for research and biopharmaceutical production. The HEK Host Cell Proteins (HCP) kit is designed to measure contaminants originating from the HEK cells used to manufacture therapeutic antibodies. This kit can be utilized to quantify HEK HCP proteins at various stages, from routine bioprocess operations to crude harvest materials to final products. Our simple procedure and robust assay offer increased throughput compared to ELISA.
Formats
- Our 100 assay point kit allows you to run 100 wells in 96-well format, using a 50 µL reaction volume (5 µL of sample).
- Our 500 assay point kit allows you to run 500 wells in 96-well or 384-well format, using a 50 µL reaction volume (5 µL of sample).
- Our 5,000 assay point kit allows you to run 5,000 wells in 96-well or 384-well format, using a 50 µL reaction volume (5 µL of sample).
Features
- No-wash steps, no separation steps
- ELISA alternative technology
- Sensitive detection
- Broad sample compatibility
- Small sample volume
- Results in less than 4 hours
- Half the time of an ELISA assay
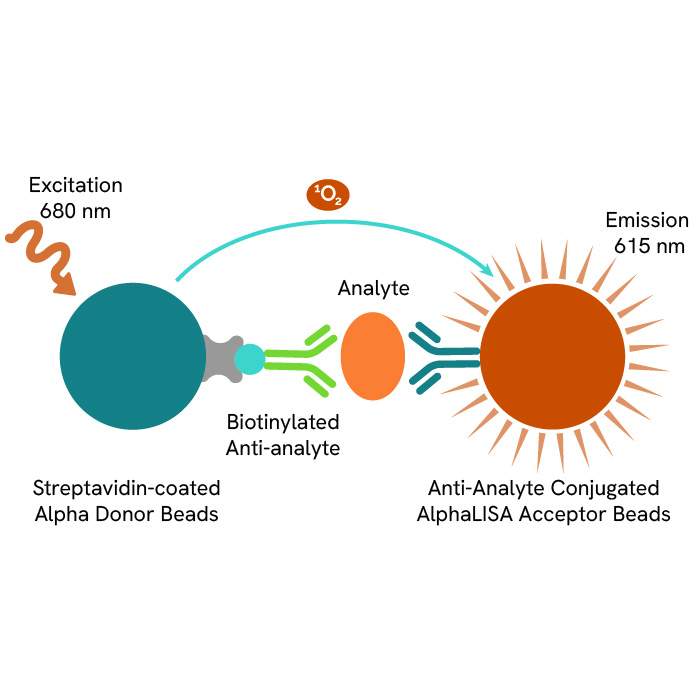
AlphaLISA technology allows the detection of molecules of interest in a no-wash, highly sensitive, quantitative assay. In an AlphaLISA assay, a biotinylated anti-analyte antibody binds to the Streptavidin-coated Donor beads while another anti-analyte antibody is conjugated to AlphaLISA Acceptor beads. In the presence of the analyte, the beads come into close proximity. The excitation of the Donor beads causes the release of singlet oxygen molecules that triggers a cascade of energy transfer in the Acceptor beads, resulting in a sharp peak of light emission at 615 nm.

AlphaLISA CHO HCP Detection Kit, 100 Assay Points
CHO cells are widely used expression hosts for recombinant proteins and are utilized for the generation of monoclonal antibodies. The CHO Host Cell Proteins (HCP) kit is designed to measure contaminants originating from the CHO cells used to manufacture therapeutic antibodies. This kit can be utilized to quantify CHO HCP proteins at various stages, from routine bioprocess operations to crude harvest materials to final products. Our simple procedure and robust assay offer increased throughput compared to ELISA.
Formats:
- Our 100 assay point kit allows you to run 100 wells in 96-well format, using a 50 µL reaction volume (5 µL of sample).
- Our 500 assay point kit allows you to run 500 wells in 96-well or 384-well format, using a 50 µL reaction volume (5 µL of sample).
- Our 5,000 assay point kit allows you to run 5,000 wells in 96-well or 384-well format, using a 50 µL reaction volume (5 µL of sample).
Features:
- No-wash steps, no separation steps
- ELISA alternative technology
- Sensitive detection
- Broad sample compatibility
- Small sample volume
- Results in less than 3 hours
- Half the time of an ELISA assay
AlphaLISA technology allows the detection of molecules of interest in a no-wash, highly sensitive, quantitative assay. In an AlphaLISA assay, a biotinylated anti-analyte antibody binds to the Streptavidin-coated Donor beads while another anti-analyte antibody is conjugated to AlphaLISA Acceptor beads. In the presence of the analyte, the beads come into close proximity. The excitation of the Donor beads causes the release of singlet oxygen molecules that triggers a cascade of energy transfer in the Acceptor beads, resulting in a sharp peak of light emission at 615 nm.
Protein contamination detection technology
To aid rapid protein contamination detection, utilize an automated microfluidic analysis platform that can support FDA 21 CFR Part 11 regulations. By providing quantitative results for contaminants while using minimal sample volumes, the LabChip™ protein characterization system is invaluable in the biopharmaceutical manufacturing workflow by significantly enhancing protein purity assessment and helping you realize higher quality standards.

LabChip GXII Touch HT Protein Characterization System
LabChip GXII Touch instrument simplifies sample analysis
User friendly operation:
- Load sample plate and chip
- Select samples (up to 384 in a run)
- Select assay type
- Touch 'Run' to start
- Automatically export data directly to your network or LIMS system
Observe runs in real time:
- Sample analysis in as few as 42 seconds
- View electropherogram in real time during data collection
- Overlay collected data to compare sample profiles
- Select from various run time analytical feature annotations
See data in real time or export for later analysis:
- Choose "display" in E-gram, virtual gel, or data table format (Figure 1)
- Pull multiple archived plates into data review or analytical comparisons
- Apply data mining filter functions on key attributes
- Highlight expected peaks
- Track relevant user access and data history parameters with FDA 21 CFR Part 11 compatible software
Easy set-up for protein and nucleic acid characterization
The LabChip GXII Touch protein characterization system operator controls are designed to allow users to easily set up and execute a run in as few as three easy steps. Run templates can also be imported with the operator control features. Run templates can include well selections, sample names, peak tables, which facilitate operator ease of use, and data can be automatically exported to network or LIMS directories for subsequent analysis. Every instrument comes with a full software package for data review, allowing analysis from current or archived data sets.
Designed to facilitate integration of quality by design initiatives into workflow
The LabChip GXII Touch protein characterization system offers rapid quantification and quality control throughout biotherapeutics and genomic workflows. For example, automating the characterization process allows multiple critical quality attributes to be obtained significantly faster. Researchers can now screen for optimal protein characteristics earlier in the process and integrate Quality by Design initiatives into their biotherapeutics development workflow.
21CFR Part 11 compatible software is available with the LabChip GXII Touch protein characterization system
LabChip GXII Touch and Reviewer Software contain built-in technical controls and features specifically designed to support FDA 21 CFR Part 11 regulations. These features include a shared user account database, access controls, device check, enforced sequencing of run steps, audit trails, record copying, record retention, system documentation, and electronic signature controls.
To assist in your compliance planning, LabChip GxP Security Software is available which allows the LabChip GXII Touch protein characterization system to run in an environment consistent with CFR21 Part 11 compliance and validation (Figure 2). This includes access security, data security and verification, and a complete set of audit logging functionality.
LabChip CE: How does it work?
LabChip CE is performed on a small, microfluidic chip. Prior to analysis, reagents are loaded into the individual wells of the chip. These wells are connected to tiny microchannels about the size of a human hair etched within the quartz microfluidic chip. When the chip is loaded into the LabChip GXII Touch protein characterization system, the chip's wells interface with platinum electrodes that provide voltage and current control. The instrument moves the microtiter plate directly under the chip's capillary 'sipper', and approximately 150 nL of sample is aspirated onto the chip. Sample staining and destaining are performed automatically on the instrument platform. Individual sample analytes are separated electrophoretically and the bands detected via laser induced fluorescence in the capillaries of the chip. Sizing and concentration for each band are determined using ladder and internal markers. Rinsing the sipper between samples minimizes cross contamination.