
AutoDELFIA free hCGß Kit
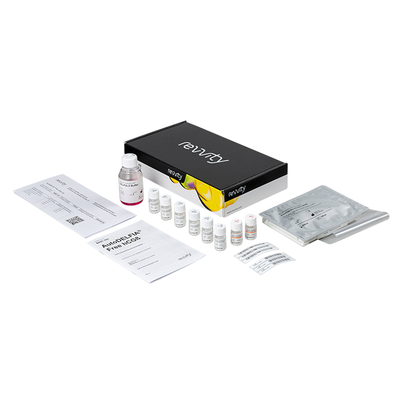
AutoDELFIA free hCGß Kit
AutoDELFIA free hCGß Kit
| Feature | Specification |
|---|---|
| Application | Prenatal screening |
| Disorders | Common Trisomies |
Product variant
Unit Size: 96 assays
Part #:
B097-101
For in vitro diagnostic use. This product is only available where licensed in accordance with law. Please contact your local representative for availability.

AutoDELFIA free hCGß Kit

AutoDELFIA free hCGß Kit
AutoDELFIA free hCGß Kit

Product information
Overview
Typically, the level of the free hCGß subunit is significantly increased in maternal serum during both the first and the second trimesters of pregnancy if the fetus is affected by Down´s syndrome. These solid phase, two-site fluoroimmunometric assays are based on the direct sandwich technique in which two monoclonal antibodies are directed against two separate antigenic determinants on the free hCGß molecule.
Analytical sensitivity is typically better than 0.2 ng/mL.
Specifications
| Application |
Prenatal screening
|
|---|---|
| Brand |
AutoDELFIA®
|
| Detection Modality |
Time-Resolved Fluorescence (TRF)
|
| Disorders |
Common Trisomies
|
| Instrument Compatibility |
AutoDELFIA
|
| Regulatory Status |
CE-IVD marked (Notified Body 0537)
|
| Sample Type |
Serum
|
| Technology |
DELFIA
|
| Unit Size |
96 assays
|
Resources
Are you looking for resources, click on the resource type to explore further.
Brochure
DELFIA Prenatal Screening Solutions Brochure
Brochure for DELFIA prenatal screening solutions and assays including traditional biochemical screening, pre-eclampsia


How can we help you?
We are here to answer your questions.






























