Customer experiences
"We are using model chemagic™ 360 (Automated Nucleic Acid Extraction system) installed in our laboratory for COVID-19 testing. We are running 32 min. short protocol (recently updated by the chemagen Team) and getting good result with our RT PCR. Due to this short protocol we are able to run approx. 4000 samples / day with two instruments, The instrument supplied has been functioning well, satisfying the requirements of our applications and meeting our expectations for quality and reliability of the data generated." Department of Microbiology, Government of Rajasthan, S.M.S. Medical School, Jaipur"
"We are using model chemagic™ 360 (Automated Nucleic Acid Extraction system) installed in our laboratory for COVID-19 testing. We are running 32 min. short protocol (recently updated by the chemagen Team) and getting good result with our RT PCR. Due to this short protocol we are able to run approx. 4000 samples / day with two instruments, The instrument supplied has been functioning well, satisfying the requirements of our applications and meeting our expectations for quality and reliability of the data generated." Department of Microbiology, Government of Rajasthan, S.M.S. Medical School, Jaipur"
"We had good earlier experience with the chemagic 360 nucleic acid extraction system and found it to be highly versatile, filling many existing and future needs in our operations. When the viral extraction kit and RT-PCR kit became available it was a natural choice to adopt the complete workflow from a single, reliable and local supplier."
Biopsense, Jyväskylä, Finland
SARS-CoV-2 RNA detection
With an ultrafast run-time of 18 min, SARS-CoV-2 RNA extraction is performed from plasma, swabs or saliva in the high-throughput workflow on the chemagic 360 instrument. Please find below an interesting example of use.
With an ultrafast run-time of 18 min, SARS-CoV-2 RNA extraction is performed from plasma, swabs or saliva in the high-throughput workflow on the chemagic 360 instrument. Please find below an interesting example of use.
PCR detection of microorganisms
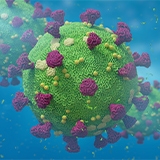
Detect diverse microorganisms from a wide range of sample material including stool, whole ticks, and body fluids using the chemagic Viral NA/gDNA 200 Kit H96 for low volumes and the chemagic Body Fluid 1k Kit H96 (CMG-1131) for high volumes. The chemagic Viral DNA/RNA 300 Kit H96 (CMG-1033-S) can be employed for clear body fluids such as plasma, serum, swabs, and saliva.
Detect diverse microorganisms from a wide range of sample material including stool, whole ticks, and body fluids using the chemagic Viral NA/gDNA 200 Kit H96 for low volumes and the chemagic Body Fluid 1k Kit H96 (CMG-1131) for high volumes. The chemagic Viral DNA/RNA 300 Kit H96 (CMG-1033-S) can be employed for clear body fluids such as plasma, serum, swabs, and saliva.
Compatible with third party PCR providers (including the EURORealTime™ PCR Systems from EUROIMMUN), these workflows are regularly employed in demanding, high throughput workflows in research and diagnostic labs around the world.
Rapid Candida auris detection with real-time PCR
Candida auris genetic testing is crucial for identifying and tracking the spread of this multidrug-resistant fungal pathogen, aiding in infection control measures and guiding appropriate antifungal treatment strategies.
Revvity has developed a C. auris detection real-time PCR workflow that uses the chemagic™ Pathogen NA Kit H96 (CMG-1033-G) on the chemagic 360 instrument for high throughput nucleic acid extraction from skin swabs, environmental surface swabs, or laboratory cultures.
Candida auris genetic testing is crucial for identifying and tracking the spread of this multidrug-resistant fungal pathogen, aiding in infection control measures and guiding appropriate antifungal treatment strategies.
Revvity has developed a C. auris detection real-time PCR workflow that uses the chemagic™ Pathogen NA Kit H96 (CMG-1033-G) on the chemagic 360 instrument for high throughput nucleic acid extraction from skin swabs, environmental surface swabs, or laboratory cultures.
SARS-CoV-2 wastewater surveillance
Wastewater testing for SARS-CoV-2 RNA can provide real-time surveillance of viral spread at the community level. Due to large sample volumes, sample inhomogeneity and continuous flow, special pre-treatment and concentration of samples are often required e.g. with the use of filters11 or by direct extraction12.
Wastewater testing for SARS-CoV-2 RNA can provide real-time surveillance of viral spread at the community level. Due to large sample volumes, sample inhomogeneity and continuous flow, special pre-treatment and concentration of samples are often required e.g. with the use of filters11 or by direct extraction12.

Cost-effective multiplex testing of tick-borne pathogens
Tick-borne pathogens are difficult to detect due to poor sensitivity and the lack of comprehensive tick-borne panels to cover pathogens endemic to different regions.
With the chemagic Viral NA/gDNA 200 Kit H96 (CMG-1049-A), extractions can be performed from blood, synovial fluid or whole ticks.
Tick-borne pathogens are difficult to detect due to poor sensitivity and the lack of comprehensive tick-borne panels to cover pathogens endemic to different regions.
With the chemagic Viral NA/gDNA 200 Kit H96 (CMG-1049-A), extractions can be performed from blood, synovial fluid or whole ticks.
Featured products




References:
- MacKay, M.J., Hooker, A.C., Afshinnekoo, E. et al. The COVID-19 XPRIZE and the need for scalable, fast, and widespread testing. Nat Biotechnol 38, 1021--1024 (2020).
- FDA, "SARS-CoV-2 Reference Panel comparative Data" (15th September, 2020)
- Ludwig, K.U., Schmithausen, R.M., Li, D. et al. LAMP-Seq enables sensitive, multiplexed COVID-19 diagnostics using molecular barcoding. Nat Biotechnol 39, 1556--1562 (2021).
- Siedner, M.J., Moorhouse, M.A., Simmons, B. et al. Reduced efficacy of HIV-1 integrase inhibitors in patients with drug resistance mutations in reverse transcriptase. Nat Commun 11, 5922 (2020).
- Kleines M, Schellenberg K, Ritter K. Efficient extraction of viral DNA and viral RNA by the Chemagic viral DNA/RNA kit allows sensitive detection of cytomegalovirus, hepatitis B virus, and hepatitis G virus by PCR. J Clin Microbiol. 41(11):5273-5276. (2023)
- Bliss, Jesse et al. "High Prevalence of Shigella or Enteroinvasive Escherichia coli Carriage among Residents of an Internally Displaced Persons Camp in South Sudan." The American journal of tropical medicine and hygiene vol. 98,2: 595-597.(2018)
- Michel J, Targosz A, Rinner T, et al. Evaluation of 11 commercially available PCR kits for the detection of monkeypox virus DNA (2022). Euro Surveill. 27(45):2200816 (2022)
- Tang YW, Lozano L, Chen X, et al. An Isothermal, Multiplex Amplification Assay for Detection and Genotyping of Human Papillomaviruses in Formalin-Fixed, Paraffin-Embedded Tissues. J Mol Diagn 22(3):419-428 (2020)
- Rajeevan MS, Patel S, Li T, Unger ER. NanoString Technology for Human Papillomavirus Typing. Viruses. 13(2):188. (2021)
- Lefèvre AC, Pallisgaard N, Kronborg C, Wind KL, Krag SRP, Spindler KG. The Clinical Value of Measuring Circulating HPV DNA during Chemo-Radiotherapy in Squamous Cell Carcinoma of the Anus. Cancers (Basel). 2021;13(10):2451. Published 2021 May 18.
- LaTurner ZW, Zong DM, Kalvapalle P, et al. Evaluating recovery, cost, and throughput of different concentration methods for SARS-CoV-2 wastewater-based epidemiology. Water Res. 197:117043. (2021)
- West NW, Vasquez AA, Bahmani A, et al. Sensitive detection of SARS-CoV-2 molecular markers in urban community sewersheds using automated viral RNA purification and digital droplet PCR. Sci Total Environ. 847:157547 (2022)