

HTRF Human and Mouse Total IRS1 Detection Kit, 500 Assay Points







| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |









Product information
Overview
The HTRF total IRS1 assay is designed to be used as a normalization assay with our Phospho-IRS1 kits. Total IRS1 assay is optimal to monitor insulin resistance. IRS1 is a relevant marker for insulin resistance, tumor development, and metastatic progression. It is associated with type 2 diabetes, obesity, and cancer.
How it works
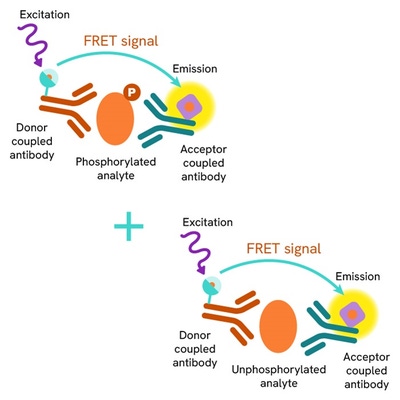
Total-IRS1 assay principle
The total IRS1 assay quantifies the expression level of IRS1 in a cell lysate. Contrary to Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis or transfer. The total IRS1 assay uses two labeled antibodies: one coupled to a donor fluorophore, the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In presence of IRS1 in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample, and provides a means of assessing the proteins expression under a no-wash assay format.

Total-IRS1 2-plate assay protocol
The 2 plate protocol involves culturing cells in a 96-well plate before lysis then transferring lysates to a 384-well low volume detection plate before adding Total IRS1 HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Total-IRS1 1-plate assay protocol
Detection of total IRS1 with HTRF reagents can be performed in a single plate used for culturing, stimulation and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
Total IRS1 assay validation on C2C12 mouse myotubes
C2C12 cells were cultured until 100% confluency was reached. They were then cultured for 7 days in 2% horse serum media to induce the differentiation. After a starvation step in serum-free medium, cells were treated with a cocktail of pro-inflammatory cytokines and/or Insulin. After cell supernatant removal, cells were lysed with 1.5 mL of supplemented lysis buffer #1 for 30 min at RT under gentle agitation. 16 µL of lysate were then transferred into a 384-well low volume white microplate for HTRF detection using the phospho- and total IRS1 kit reagents.


Total IRS1 assay validation on 3T3-L1 mouse adipocytes
3T3-L1 cells were cultured in medium with 10% newborn calf serum. They were then cultured for 7 days in medium containing 10% FCS, insulin, dexamethasone, IBMX and µM thiazolidinedione to induce differentiation from fibroblasts to adipocytes. After an overnight starvation step in serum-free medium, the cells were treated with 100 nM insulin for 45 min or with a cocktail of pro-inflammatory cytokines for 30 min. After cell supernatant removal, cells were lysed and transferred into a 384-well low volume white microplate for HTRF detection using the phospho- and total IRS1 kit reagents (Samples were kindly provided by JF Tantis research team, UMR INSERM U1065/UNS, C3M, Nice, France).

Total IRS1 assay validation on MCF-7 human breast cancer cells
50,000 human breast cancer MCF-7 cells were plated in a 96-well plate in complete culture medium, and incubated for 24h at 37°C, 5% CO2. Increasing concentrations of Insulin were added and incubated for 45 min at 37°C. After cell culture medium removal, cells were lysed with 50 µL of lysis buffer for 30 min at RT under gentle shaking. 16 µL of lysate were then transferred into a 384-well sv white microplate for detection of phospho-IRS1 Ser 312 and total IRS1, using 4 µL of the HTRF detection reagents.
Phospho-IRS1 assay validation on MCF-7 human breast cancer cells
50,000 human breast cancer MCF-7 cells were plated in a 96-well plate in complete culture medium, and incubated for 24h at 37°C, 5% CO2. Increasing concentrations of Insulin were added and incubated for 45 min at 37°C. After cell culture medium removal, cells were lysed with 50 µL of lysis buffer for 30 min at RT under gentle shaking. 16 µL of lysate were then transferred into a 384-well sv white microplate for detection of phospho-IRS1 Ser 312 and total IRS1, using 4 µL of the HTRF detection reagents.

Simplified pathway
IRS1/PI3K/AKT signaling pathway

Modulation of the IRS1/PI3K/AKT signaling pathway
Inhibition of the pathway. While tyrosine-phosphorylated IRS-1 positively regulates insulin signaling, serine phosphorylation generally serves as negative feedback to down-regulate IRS-1 function. Increased serine phosphorylation of IRS-1 represents a hallmark of insulin resistance in adipose tissue and skeletal muscles. Binding of Insulin or IGF-1 to the extracellular a-subunits of the IR or IGF-1R triggers autophosphorylation of the transmembrane ß-subunits of the receptor, thus activating it. IRS1/AKT pathway activation plays a critical role in maintaining glucose homeostasis. AKT also activates mTOR that mediates cell growth and survival, as well as protein synthesis. IRS1 downregulation is a relevant diabetic marker while overexpression is associated with metastatic growth and cancers.
Activation of the pathway. Binding of Insulin or IGF-1 to the extracellular a-subunits of the IR or IGF-1R (or to hybrids of both receptors) triggers autophosphorylation of the transmembrane ß-subunits of the receptor. IRS-1 is then recruited by the activated tyrosine kinase receptor and phosphorylated on multiple tyrosine residues, creating a docking site for PI3K at the membrane. The PI3K pathway is then activated with downstream phosphorylations of AKT, followed by AS160, GSK3 and FoxO1, leading respectively to GLUT4 translocation/glucose uptake, glycogen synthesis and inhibition of gluconeogenesis. Thus, the IRS1/AKT pathway activation plays a critical role in maintaining glucose homeostasis. AKT also activates mTOR that mediates cell growth and survival, as well as protein synthesis.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Metabolism/Diabetes
NASH/Fibrosis
|
| Unit Size |
500 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.
This flyer details HTRF™ and AlphaLISA™ assays for investigating key biomarkers and signaling pathways in metabolic disease...
Obesity is a complex condition characterized by excessive fat accumulation, posing significant health and socioeconomic challenges...


Loading...
How can we help you?
We are here to answer your questions.






























