HTRF Human and Mouse Phospho-FOXO1 (Ser256) Detection Kit, 500 Assay Points






| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |









Product information
Overview
The phospho-FoxO1 (Ser256) assay is designed for the robust quantification of FoxO1 modulation, phosphorylated on Ser256, as an indicator of its inactivation. FoxO1 is a member of the Forkhead transcription factor FOXO subfamily, and is highly expressed in insulin-responsive tissues where it regulates glucose/lipid metabolism and stress resistance. It also functions as a tumor suppressor by inhibiting cell proliferation. FoxO1 deregulation plays a critical role in the development of metabolic disorders such as diabetes and NAFLD. Its dysfunction is also linked to various types of cancer.
How it works
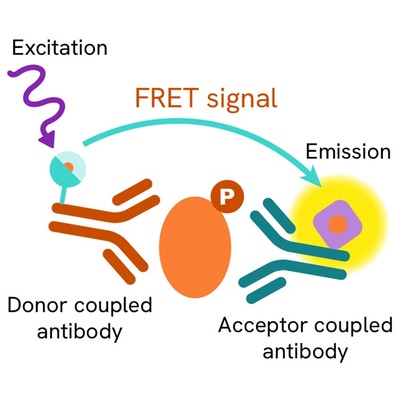
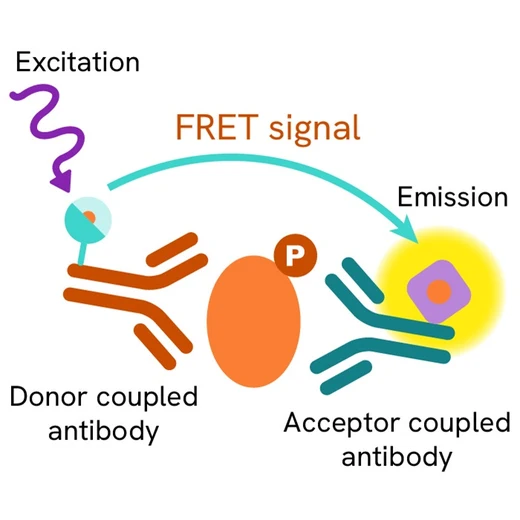
Phospho-FoxO1 (Ser251) assay principle
The Phospho-FoxO1 (Ser251) assay measures FoxO1 when phosphorylated at Ser251. Contrary to Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis or transfer. The Phospho-FoxO1 (Ser251) assay uses 2 labeled antibodies: one with a donor fluorophore, the other one with an acceptor. The first antibody is selected for its specific binding to the phosphorylated motif on the protein, the second for its ability to recognize the protein independent of its phosphorylation state. Protein phosphorylation enables an immune-complex formation involving both labeled antibodies and which brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample, and provides a means of assessing the proteins phosphorylation state under a no-wash assay format.

Phospho-FoxO1 (Ser251) 2-plate assay protocol
The 2 plate protocol involves culturing cells in a 96-well plate before lysis then transferring lysates to a 384-well low volume detection plate before adding Phospho-FoxO1 (Ser251) HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Phospho-FoxO1 (Ser251) 1-plate assay protocol
Detection of Phosphorylated FoxO1 (Ser251) with HTRF reagents can be performed in a single plate used for culturing, stimulation and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
FoxO1 Inactivation following insulin treatment
Huma HepG2 and mouse C2C12 cells were plated and cultured in a high-glucose medium prior to treatment with increasing concentrations of insulin for 1 hour. After lysis, cells were transferred twice over into a low volume white microplate finally before before the additon of the HTRF phospho-FoxO1 or total FoxO1 detection antibodies. The HTRF signal was recorded after 4 hours of incubation. In both cell lines, insulin treatment induces FoxO1 phosphorylation on Ser256 (inactivation), while its expression level remains stable.


FoxO1 activation in HepG2 cells with cytokines and oxidative stress
Human HepG2 cells were plated and cultured in high-glucose culture medium before being treated with a cocktail of TNF-a/IL-1ß/IL-6 or H2O2. Following lysis, soluble cell lysates were transferred twice over into a low volume white microplate before finally adding HTRF phospho-FoxO1 or total FoxO1 detection antibodies. Results indicate pro-inflammatory cytokines and ROS (reactive oxygen species) are factors driving metabolic deregulation by inducing FoxO1 dephosphorylation (activation) in HepG2 cell line, while its expression level remains stable.


FoxO1 Activaiton using PI3K inhibitor Wortmannin in pancreatic ß-cells
Mouse pancreatic ß-cell line Min-6 was plated and cultured in high-glucose culture medium for 3 days prior to treatment with increasing concentrations Wortmannin for 1 hour. Following lysis, cell lysates were transferred twice over into a low volume white microplate before finally adding HTRF phospho-FoxO1 or total FoxO1 detection antibodies. Results showed Wortmannin induces PI3K/AKT pathway inhibition, leading to FoxO1 dephosphorylation (activation) in Min-6 cell line. The FoxO1 expression level remains stable, demonstrating that there is no cytotoxic effect of the compound on the cells.

Inactivation of FoxO1 using IGF-1 in cancer cervical cell line HeLa
Human HeLa cells were plated and cultured in complete medium and incubated for 24. They were then incubated overnight in serum-free medium before treatment with increasing concentrations of IGF-1. Folowing lysis, cell lysates were transferred twice over into a low volume white microplate before the addition of phospho or total FoxO1 antibodies. Results show that the growth factor IGF-1 induces FoxO1 inactivation by phosphorylation on Ser256, leading to tumor cell proliferation and that it's expression level remains stable.

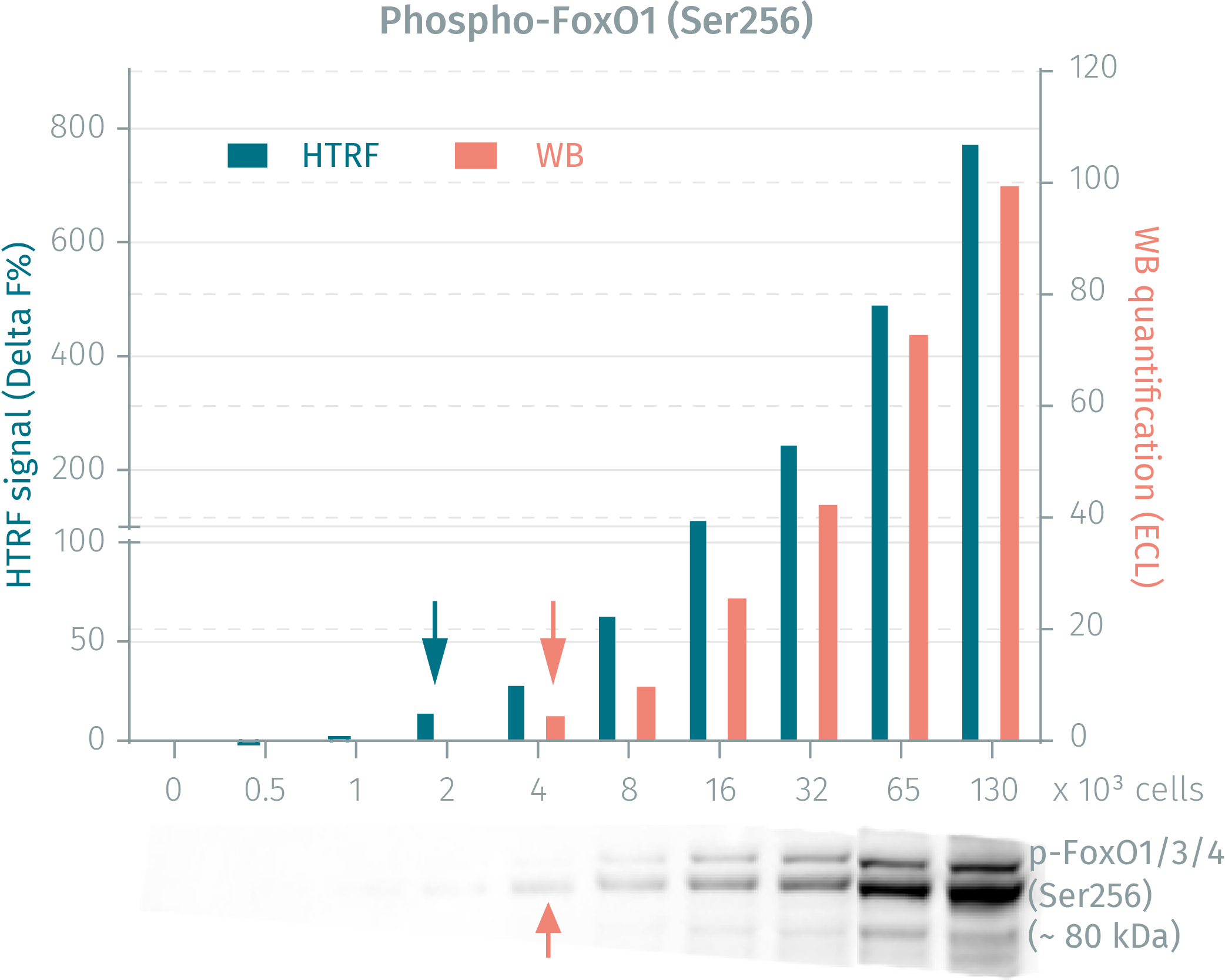
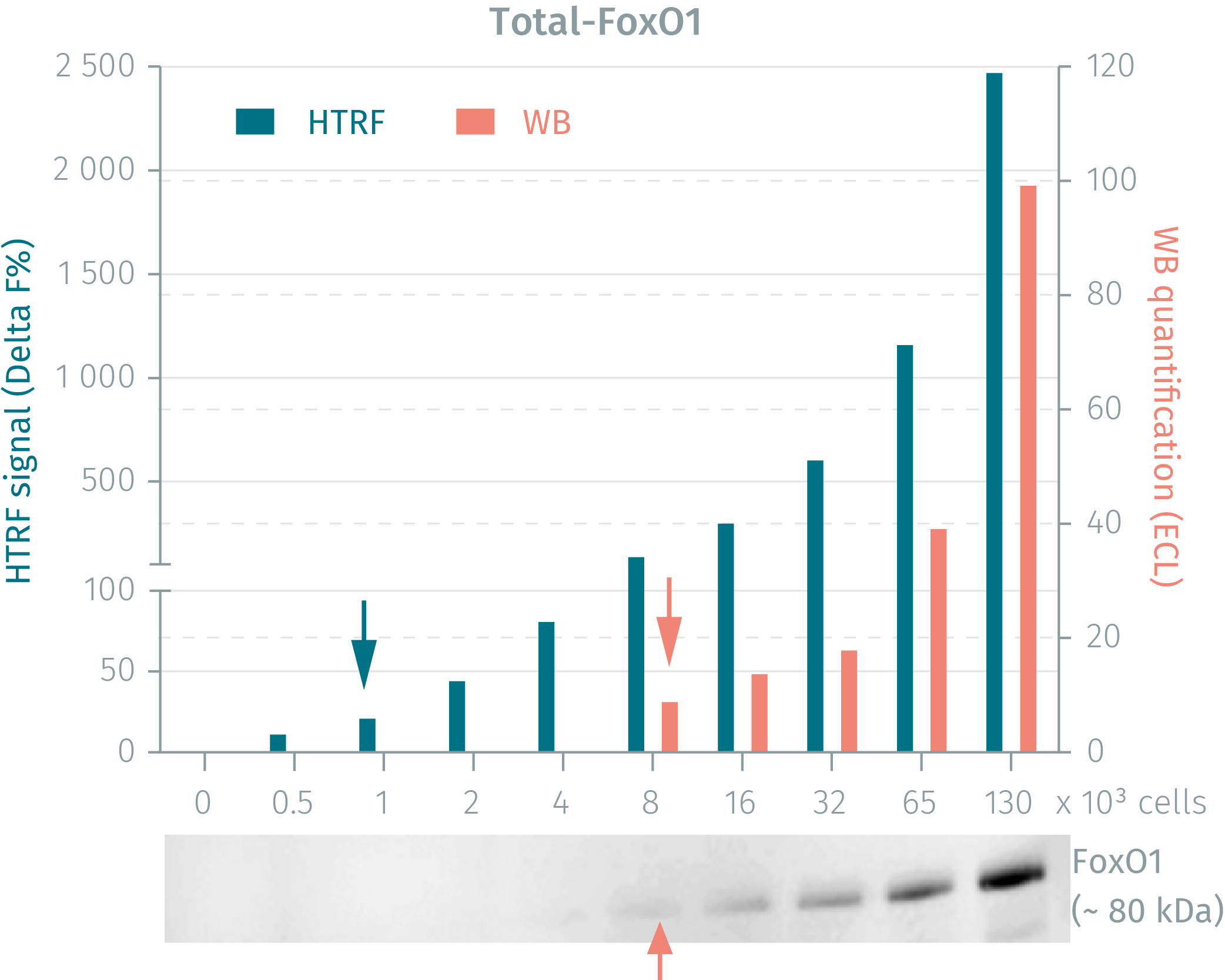
HTRF phospho- & total FoxO1 assays vs WB on human HEK293 cells
HEK293 cells were seeded and cultured in complete medium until 90% confluency was reached. Following lysis, soluble supernatants were collected via centrifugation. Serial dilutions of the cell lysate were performed and transferred into a low volume white microplate before the addition of HTRF phospho- or total detection antibodies. Equal amounts of lysates were used for a side by side comparison between WB and HTRF. The HTRF assay is shown to be 2-fold more sensitive than the Western Blot technique. Using the HTRF Total FoxO1 kit the HTRF assay is 8-fold more sensitive than the WB.
Simplified pathway
FoxO1 Simplified Pathway
The Insulin/IGF-1 signaling pathway activates the AKT kinase which in turn phosphorylates FoxO1 on Ser256, leading to its cytoplasmic sequestration and the inhibition of its transcriptional activity. In these conditions, hepatic glucose production is inhibited, lipogenesis increases, and cells proliferate. Conversely, the dephosphorylated form of FoxO1 can translocate to the nucleus and induce the transcription of genes involved in glucose production, lipolysis, inhibition of lipogenesis, stress resistance, apoptosis, autophagy and inflammation. The stress proteins JNK and p38 activate FoxO1 in the presence of pro-inflammatory cytokines as well as oxidative stress, fasting and exercise.

Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
Lysis Buffer 4
Lysis Buffer 5
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Metabolism/Diabetes
NASH/Fibrosis
Oncology & Inflammation
|
| Unit Size |
500 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
HTRF™ Application Note
Non-alcoholic fatty liver disease (NAFLD) includes a range of liver conditions unrelated to alcohol...
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.
This flyer details HTRF™ and AlphaLISA™ assays for investigating key biomarkers and signaling pathways in metabolic disease...
Obesity is a complex condition characterized by excessive fat accumulation, posing significant health and socioeconomic challenges...


Loading...
How can we help you?
We are here to answer your questions.