

HTRF Human and Mouse Phospho-Ubiquitin (Ser65) Detection Kit, 500 Assay Points






This HTRF kit enables the cell-based quantitative detection of phosphorylated Ubiquitin at Ser65, as a readout of Mitochondrial stress and Mitophagy activation.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
This HTRF kit enables the cell-based quantitative detection of phosphorylated Ubiquitin at Ser65, as a readout of Mitochondrial stress and Mitophagy activation.









Product information
Overview
The Phospho-Ubiquitin (Ser65) Cellular assay is designed to monitor the level of the phosphorylated form of the Ubiquitin chain. Ubiquitin itself is a 76 amino acid protein (8.5 kDa) that is ubiquitously expressed and highly conserved, with 100% AA sequence identity between human and mouse.
Phosphorylation of Ubiquitin is a key step in the process of mitochondria quality control through the Parkin/PINK-1 interaction. Parkin is a Ubiquitin ligase E3 that conjugates Ubiquitin to impaired-mitochondrial proteins, leading to organelle degradation. PINK1 is a Ser/Thr kinase that accumulates only on impaired mitochondria and phosphorylates two substrates, the Ubiquitin-like domain of Parkin and Ubiquitin chains.
The amplified Parkin/PINK-1 dependent Ubiquitin functions act as a signal for the sequestration and degradation of damaged mitochondria (mitophagy). Dysregulation of this signaling is linked to some forms of Parkinson's Disease.
Depolarization of mitochondria by uncoupling agents, such as CCCP and valinomycin, causes PINK1 to accumulate on the outer mitochondrial membrane and trigger PhosphoS65-Ubiquitin production.
How it works
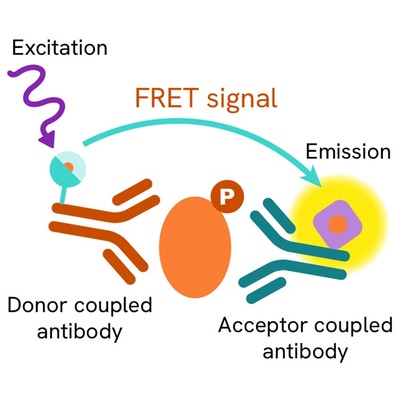
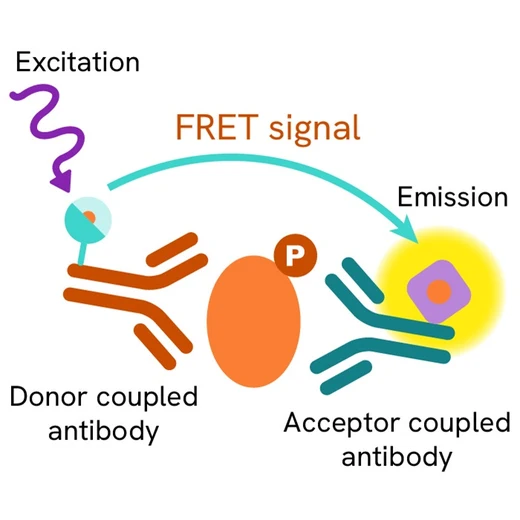
Phospho-Ubiquitin (Ser65) assay principle
The Phospho-Ubiquitin (Ser65) assay measures Ubiquitin when phosphorylated at Ser65. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The assay uses 2 antibodies, one labeled with a donor fluorophore and the other with an acceptor. The first antibody was selected for its specific binding to the phosphorylated motif on the protein, the second for its ability to recognize the protein independently of its phosphorylation state. Protein phosphorylation enables an immune-complex formation involving both labeled antibodies, and which brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample, and provides a means of assessing the protein's phosphorylation state under a no-wash assay format.

Phospho-Ubiquitin (Ser65) two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates to a 384-well low volume detection plate before the addition of Activation Buffer followed by Phospho-Ubiquitin (Ser65) HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Phospho-Ubiquitin (Ser65) one-plate assay protocol
Detection of Phosphorylated Ubiquitin (Ser65) with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
CCCP stimulation curve on human neuronal cell line
Neuroblastoma (human) cells were plated (200,000 cells/well) in a 96 well plate and cultured overnight in complete culture medium, 37°C, 5% CO2.
The day after, medium was renewed and the cells were incubated for 6h at 37°, 5% CO2, with increasing concentrations of Carbonyl cyanide m-chlorophenyl-hydrazone (CCCP), a mitochondrial uncoupler. After medium removal, the cells were lysed with 50 µL of 1X supplemented lysis buffer #1 for 30 min at RT under gentle shaking, and 14 µL of lysate were transferred into a low volume white microplate before the addition of 2µL of Activation Buffer, followed by 4 µL of the HTRF Ubiquitin Phospho-S65 detection antibodies.
HTRF Tubulin assay was run simultaneously on 4µL of ¼ diluted lysates as a normalization tool. The HTRF signal was recorded after an overnight incubation.
In neuronal cells, CCCP promoted Ubiquitin phosphorylation on Ser65. The EC50 value agreed with the reported value. The tubulin assay shows that there is no CCCP toxicity issue within the concentration range.

CCCP stimulation curve on mouse Neuro-2A cell line
Neuro-2A (mouse) cells were plated (200,000 cells/well)in a 96 well plate and stimulated with CCCP as described above for neuronal cells. Then 14 µL of lysate were transferred into a low volume white microplate before the addition of 2µL of Activation Buffer, followed by 4 µL of the HTRF Ubiquitin Phospho-S65 detection antibodies. HTRF Tubulin assay was run simultaneously on 4µL neat lysates.
The HTRF signal was recorded after an overnight incubation.
In Neuro-2A cells, CCCP promoted Ubiquitin phosphorylation on Ser65. The EC50 is in the micromolar range, similar to the value observed for neuronal cells.

Valinomycin stimulation curve on human neuronal cell line
Neuronal (human) cells were plated (200,000 cells/well) in a 96 well plate and cultured overnight in complete culture medium, 37°C / 5% CO2.
The day after, medium was renewed and the cells were incubated for 4h at 37°C / 5% CO2 with increasing concentrations of valinomycin, a mitochondrial uncoupler. After medium removal, the cells were then lysed with 50 µL of 1X supplemented lysis buffer #1 for 30 minutes at RT under gentle shaking, and 14 µL of lysate were transferred into a low volume white microplate before the addition of 2µL of Activation Buffer, followed by 4 µL of the HTRF Ubiquitin Phospho-S65 detection antibodies. The HTRF signal was recorded after an overnight incubation. In neuronal cells, valinomycin promoted Ubiquitin phosphorylation on Ser65, with an EC50 in the nanomolar range.

HTRF Phospho-Ubiquitin (Ser65) assay compared to Western Blot
Human neuronal cells were seeded in a T175 flask in complete culture medium and incubated with 10µM CCCP for 6 h at 37°C, 5% CO2. Then the cells were lysed with 3 mL of supplemented lysis buffer#1 for 30min at RT under gentle shaking. Soluble supernatants were collected after a 10 min centrifugation. Serial dilutions of the cell lysate were performed in the supplemented lysis buffer, and 14 µL of each dilution were transferred into a 384-well low volume white microplate before the addition of 2µL Activation Buffer, followed by 4 µL of the HTRF Ubiquitin Phospho-S65 detection reagents. Equal amounts of lysates were used for a side by side comparison between Western Blot and HTRF.
This result demonstrates that the HTRF Ubiquitin phospho-S65 assay is 4-fold more sensitive than the Western Blot, at least under these experimental conditions.

Simplified pathway
In a depolarization-induced mitophagy process, PINK1 (PTEN-induced kinase 1) linked to the outer mitochondrial membrane is activated by dimerization and autophosphorylation. Once activated, PINK-1 phosphorylates Parkin, a Ubiquitin ligase, at position Ser65 of its Ubiquitin-like domain. PINK-1 also phosphorylates the poly-Ubiquitin chains linked to proteins of the outer mitochondrial membrane at position Ser65. The resulting phospho-Ubiquitin Ser65 functions as the key signal in recruiting mitochondrial proteins to initiate mitophagy.

Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
Lysis Buffer 3
Lysis Buffer 4
Lysis Buffer 5
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Neuroscience
|
| Unit Size |
500 assay points
|
Video gallery
Citations
Resources
Are you looking for resources, click on the resource type to explore further.
Studying mitophagy activation in relation to ubiquitin phosphorylation with HTRF
Recent research studies have established that...
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.
Neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, and Huntington’s, are complex disorders that affect millions...


Loading...
How can we help you?
We are here to answer your questions.






























