HTRF Human and Mouse Phospho-TBK1 (Ser172) Detection Kit, 500 Assay Points






The phospho-TBK1 (Ser172) assay enables the cell-based quantitative detection of phosphorylated TBK1 on Ser172 as a readout of the TLR3/4, cGAS-STING pathways
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
The phospho-TBK1 (Ser172) assay enables the cell-based quantitative detection of phosphorylated TBK1 on Ser172 as a readout of the TLR3/4, cGAS-STING pathways









Product information
Overview
The phospho-TBK1 cell based assay kit conveniently and accurately quantifies phosphorylated TBK1 at Ser172. Following pathogen infection, TBK1 can be activated by bacterial LPS and pathogen nucleic acids which trigger the activation of TLR3/4 and cGAS-STING signaling axis. This phospho-TBK1 assay can be used from basic research through to preclinical drug discovery phases and contains everything you need. It offers increased throughput compared to ELISA/WB.
HTRF assays offer many advantages over other technologies:
- Homogeneous add-and-read format
- No wash steps
- Low background
- Straightforward miniaturization from 96- or 384-well microplates to high density assay formats such as 384-well low volume and 1536-well plates
- Stable signal, providing flexibility in time of readout or size of assays
How it works
Phospho-TBK1 (Ser172) assay principle
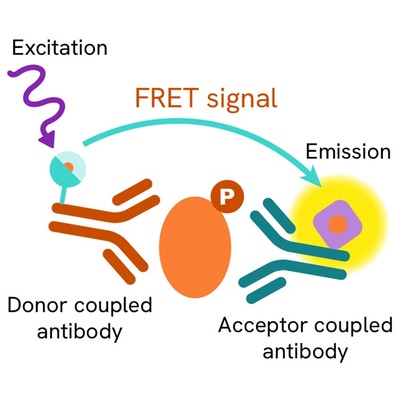
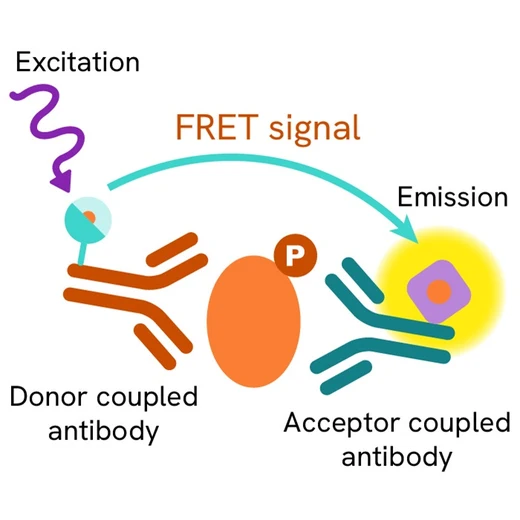
The Phospho-TBK1 (Ser172) assay measures TBK1 when phosphorylated at Ser172. Contrary to Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis or transfer. The Phospho-TBK1(Ser172) assay uses 2 labeled antibodies: one with a donor fluorophore, the other one with an acceptor. The first antibody is selected for its specific binding to the phosphorylated motif on the protein, the second for its ability to recognize the protein independent of its phosphorylation state. Protein phosphorylation enables an immune-complex formation involving both labeled antibodies and which brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample, and provides a means of assessing the proteins phosphorylation state under a no-wash assay format.

Phospho-TBK1 (Ser172) 2-plate assay protocol
The 2 plate protocol involves culturing cells in a 96-well plate before lysis then transferring lysates to a 384-well low volume detection plate before adding phospho-TBK1 (Ser172)HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Phospho-TBK1 (Ser172) 1-plate assay protocol
Detection of phosphorylated TBK1 on serine 172 with HTRF reagents can be performed in a single plate used for culturing, stimulation and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
Analysis of TBK1 phosphorylation to monitor the LPS/TLR4 axis in Kupffer cells
Mouse Kupffer cells (ImKC cell line) were plated in 96-well plates (200,000 cells/well) in complete culture medium and incubated at 37°C - 5% CO2. The day after, cells were treated for 1 hour with increasing concentrations of LPS diluted in serum-free medium. After medium removal, cells were then lysed with 50 µL of supplemented lysis buffer #4 for 30 minutes at RT under gentle shaking, and 16 µL of lysate were transferred twice over into a low volume white microplate before adding 4 µL of the HTRF® phospho-TBK1 or total TBK1 detection antibodies. The HTRF signal was recorded after an overnight incubation at RT. LPS induces the activation of TLR4 signaling, leading to a 3-fold increase in TBK1 phosphorylation on Ser172. The expression level of the kinase remains stable which demonstrates that LPS doesn't induce any cytotoxic effect.

Analysis of TBK1 phosphorylation to monitor the activation of the cGAS-STING pathway
Mouse Kupffer cells (ImKC cell line) were plated in 96-well plates (200,000 cells/well) in complete culture medium and incubated at 37°C - 5% CO2. The day after, cells were treated for 1 hour with increasing concentrations of 23-cGAMP diluted in serum-free medium. After medium removal, cells were then lysed with 50 µL of supplemented lysis buffer #4 for 30 minutes at RT under gentle shaking, and 16 µL of lysate were transferred twice over into a low volume white microplate before adding 4 µL of the HTRF® phospho-TBK1 or total TBK1 detection antibodies. The HTRF signal was recorded after an overnight incubation at RT. Cell treatment with 23-cGAMP leads to the activation of STING which in turn triggers a 3.6-fold increase in TBK1 phosphorylation on Ser172. As expected, the expression level of the kinase remains constant.

HTRF phospho- & total TBK1 cellular assays compared to Western Blot
The human cervical cancer cell line HeLa was seeded in a T175 flask in complete culture medium, and incubated for 2 days at 37°C, 5% CO2 until 90% confluency was reached. Cells were then treated with 200 nM Calyculin A for 10 minutes and lysed with 3 mL of supplemented lysis buffer #4 for 30 minutes at RT under gentle shaking. Soluble supernatants were collected after a 10-minute centrifugation. Serial dilutions of the cell lysate were performed in the supplemented lysis buffer and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of HTRF® phospho- or total TBK1 detection antibodies. Equal amounts of lysates were used for a side by side comparison between HTRF and Western Blot. Using HTRF® Phospho- or Total TBK1 kit, only 1,250 cells/well were sufficient to detect a significant signal while 2,500 cells were needed for minimal ECL signal detection using Western Blot. The HTRF assays are therefore 2 times more sensitive than the Western Blot technique.


Simplified pathway
TLR3/4 and cGAS-STING simplified pathway
TBK1 (TANK-Binding Kinase 1, also known as NAK) is a multimeric kinase activated by autophosphorylation at Ser172. This kinase is a key signaling node between several pathways leading to inflammation and autophagy. Following pathogen infection, TBK1 can be activated by bacterial LPS and pathogen nucleic acids which trigger the activation of TLR3/4 and cGAS-STING signaling axis. Upon LPS binding to TLR4 at the cell surface, the receptor translocates to endosomes and recruits the protein TRAM which in turn activates the protein TRIF. In the same way, dsRNA entering the cell binds to endosomal TLR3 which directly activates TRIF. In the case of dsDNA, it enters the cell and binds to the cytoplasmic sensor cGAS, inducing the production of 23-cGAMP which in turn activates the adaptor protein STING. Further to the activation of each of these pathways, TBK1 is recruited to different signaling complexes and activated by autophosphorylation at Ser172. The kinase in turn activates the transcription factor IRF3 which translocates to the nucleus and induces the expression of type I IFN genes, leading to inflammatory responses. TBK1 is also involved in autophagy where it directly phosphorylates the autophagy receptors optineurin and p62, which target the protein cargo to the autophagosome.

Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Cellular or Signaling Pathway |
Inflammasome/Pattern Recognition Receptors (PRRs)
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
Lysis Buffer 4
Lysis Buffer 5
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Oncology & Inflammation
|
| Unit Size |
500 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
Taking autophagy regulation research a step further
Autophagy regulation is a key molecular process involved in recycling long...
Get a useful overview of today’s immunity knowledge with this booklet
Immunity is a collection of complex processes involving...
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.
An in-depth review of molecular and cellular pathways
The maintenance of proteostasis, the biological mechanisms that control the...


Loading...
How can we help you?
We are here to answer your questions.