

HTRF Human Total STING Detection Kit, 500 Assay Points







| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |









Product information
Overview
The Total STING cellular assay monitors total STING, and can be used as a normalization assay with our phospho-STING kit. This kit is compatible with the buffers from the phospho-STING kit, so the same lysate can be used for analyses of both the phosphorylated and the total protein populations.
Following pathogen infection and binding of dsDNA to the cytoplasmic sensor cGAS, STING protein is phosphorylated by TBK1, enabling its binding to IRF3 which induces IFNs type 1 production. The STING pathway is then switched off by STING degradation involving autophagy.
In immuno-oncology, activating the STING pathway has shown promising anti-tumor effects in pre-clinical models and thus represents a therapeutic strategy to treat human cancer.
How it works
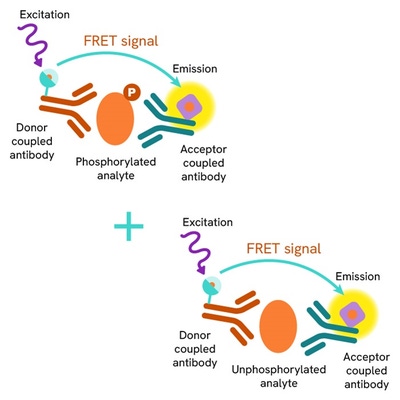
Total-STING assay principle
The Total STING assay quantifies the expression level of STING in a cell lysate. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The Total-STING assay uses two labeled antibodies, one coupled to a donor fluorophore and the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In presence of STING in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample, and provides a means of assessing the protein's expression under a no-wash assay format.

Total-STING two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates to a 384-well low volume detection plate before the addition of Total-STING HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Total-STING one-plate assay protocol
Detection of Total STING with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
2’-3’ cGAMP induces STING phosphorylation in THP1 cells
Human THP1-R232 cells (from Invivogen) were plated under 25µl in 96-well plates (400,000 cells/well) in complete culture medium. The cells were stimulated with increasing concentrations of 2’3’cGAMP (5µL for 4 hours). Cells were then lysed with the addition of 10 µL of supplemented lysis buffer #4 at 4X for 30 minutes at RT under gentle shaking. 16µL of lysate were transferred into a 384 low volume white microplate before the addition of 4 µL of the HTRF® phospho-STING or total STING detection antibodies. In parallel, Alpha-tubulin housekeeping protein was analyzed from 4µL of cell lysate, using the corresponding HTRF alpha-tubulin housekeeping kit. Finally, HTRF signals were recorded after an overnight incubation at RT.
As described elsewhere, 2’3’cGAMP induced a significant activation of the STING pathway, leading to a 6-fold increase in STING phosphorylation. The induced STING phosphorylation was associated with a down-regulation of its expression level, in agreement with autophagy mediated degradation, whereas alpha-tubulin remained stable under the same conditions.


HTRF Phospho and total STING enable accurate quantification of agonist-induced STING regulation
400,000 THP1-R232 cells (from Invivogen) were plated under 25 µL in 96-well plates in complete culture medium. The cells were stimulated with increasing concentrations of 2’3’cGAMP (5 µL for 4 hours). Cells were then lysed with the addition of 10 µL of supplemented lysis buffer #4 at 4X for 30 minutes at RT under gentle shaking. 16 µL of lysate were analyzed either by HTRF or Western Blot, in parallel.
Signal quantification by HTRF enabled accurate statistical analysis of compound effect, as shown on the graphs below, where 400 µM of cGAMP significantly decreased both total and phospho-STING (statistical method used: One way-Anova, P value <0.0001).


Total STING cellular assay compared to Western Blot
The human THP1-R232 cell line was seeded in a T175 flask in complete culture medium, and incubated for 2 days at 37°C, 5% CO2. Cells were then stimulated with 100 µM 2’3’cGAMP for 4 hours, and lysed with 3 mL of supplemented lysis buffer #4 for 30 minutes at RT under gentle shaking. Soluble supernatants were collected after a 10-minute centrifugation.
Serial dilutions of the cell lysate were performed in the supplemented lysis buffer and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of HTRF total STING detection antibodies. Equal amounts of lysates were used for a side by side comparison between HTRF and Western Blot.
Using the Total STING kit, 4,000 cells/well were enough to detect a significant signal with HTRF and Western Blot. Therefore, in our experiment the detection sensitivity of total STING protein is similar between the two methods.

Simplified pathway
STING simplified pathway
STING, for STimulator of INterferon Genes, is a cytoplasmic homodimeric protein localized in the endoplasmic reticulum which plays an essential role in innate immunity. Upon pathogen infection or mitochrondrial shrinking during apoptosis, floating dsDNAs bind and activate a DNA sensor, the cyclic GMP-AMP synthase (cGAS). Activated cGas leads to the production of 2’-3’cGAMP, a cyclic dinucleotide, which then binds to STING proteins. In turn, phosphorylated STING interacts with TANK-binding-kinase-I (TBK1), leading to the recruitment and activation of active interferon regulatory factor (IRF3) dimer. Nuclear translocation of IRF3 dimer leads to the transcription of genes encoding IFN-α/β. In addition, the STING pathway controls NF-κB dependent inflammatory cytokine expression. As a negative feedback loop, the DNA-stimulated cGAS-STING-TBK1 pathway also triggers STING protein degradation through p62 SQSTM1 associated autophagy, to switch off IFNb production.

Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
Lysis Buffer 2
Lysis Buffer 3
Lysis Buffer 4
Lysis Buffer 5
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Infectious Diseases
Oncology & Inflammation
|
| Unit Size |
500 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
Get a useful overview of today’s immunity knowledge with this booklet
Immunity is a collection of complex processes involving...
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.
Advance your autoimmune disease research and benefit from Revvity broad offering of reagent technologies


Loading...
How can we help you?
We are here to answer your questions.






























