HTRF Human Phospho-STAT6 (Tyr641) Detection Kit, 500 Assay Points






The HTRF Human Phospho-STAT6 (Tyr641) Detection Kit is an immunoassay designed for the detection and quantitation of Human STAT6 when phosphorylated at Tyr641 in cellular lysates, in a homogeneous (no-wash steps, no separation steps) format.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
The HTRF Human Phospho-STAT6 (Tyr641) Detection Kit is an immunoassay designed for the detection and quantitation of Human STAT6 when phosphorylated at Tyr641 in cellular lysates, in a homogeneous (no-wash steps, no separation steps) format.









Product information
Overview
This cell-based assay is designed to monitor the phosphorylation of STAT6 on Tyr641, which represents a hallmark of its activation.
STAT6 is a transcription factor mainly activated by the IL4 receptor, and also by the IL3, IL13 or IFNa receptor. STAT6 is phosphorylated on tyrosine 641 by Janus kinases, which leads to the formation of homodimers or heterodimers with STAT2. Finally, activated STAT6 translocates to the nucleus and mediates cytokine-induced gene expression.
HTRF assays offer many advantages over other technologies:
- Homogeneous add-and-read format
- No wash steps
- Low background
- Straightforward miniaturization from 96- or 384-well microplates to high density assay formats such as 384-well low volume and 1536-well plates
- Stable signal, providing flexibility in time of readout or size of assays
How it works
Phospho-STAT6 (Tyr641) assay principle
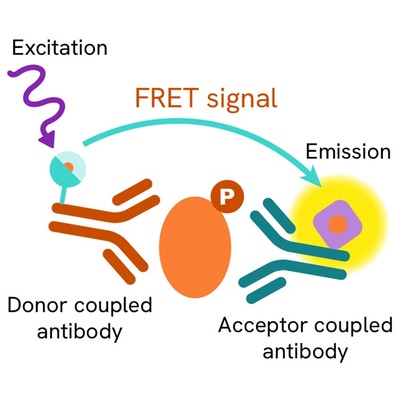
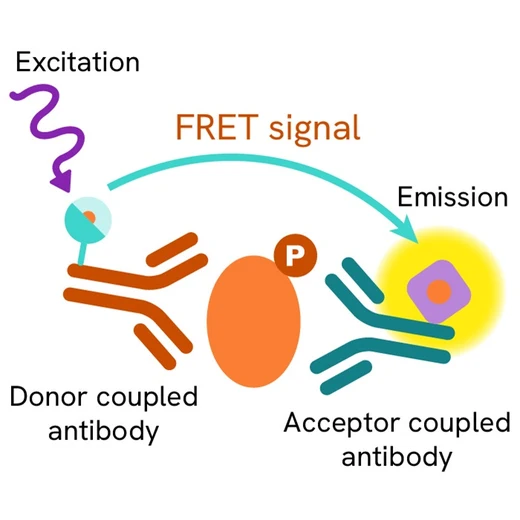
The Phospho-STAT6 (Tyr641) assay measures STAT6 when phosphorylated at Tyr641. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The Phospho-STAT6 (Tyr641) assay uses 2 labeled antibodies, one with a donor fluorophore and the other with an acceptor. The first antibody is selected for its specific binding to the phosphorylated motif on the protein, the second for its ability to recognize the protein independently of its phosphorylation state. Protein phosphorylation enables an immune-complex formation involving both labeled antibodies which brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample, and provides a means of assessing the protein’s phosphorylation state under a no-wash assay format.

Phospho-STAT6 (Tyr641) 2-plate assay protocol
The 2-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates to a 384-well low volume detection plate before the addition of Phospho-STAT6 (Tyr641) HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Phospho-STAT6 (Tyr641) 1-plate assay protocol
Detection of Phosphorylated STAT6 (Tyr641) with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
STAT6 Tyr641 phosphorylation assessed on various cell models
Various human cell lines, either adherent HeLa cells or suspensions such as TF1, U937 and TH1 cells, were seeded at 100,000 cells / well in a 96-well microplate, then stimulated with 5ng/mL of hIL4 for 20 minutes. After cell lysis with supplemented lysis buffer, 16 µL of lysate were transferred into a 384-well low volume white microplate before the addition of 4 µL of the HTRF phospho-STAT6 (Tyr641) detection reagents. The HTRF signal was recorded after an overnight incubation.

Pharmacological validation of phospho-STAT6 (Tyr641) in THP1 cells
THP1 cells were seeded at different cell densities in 96-well microplates, then stimulated with increasing concentrations of hIL4 for 20 minutes. Following the 2-plate assay protocol, 16 µL of lysate were transferred into a 384-well low volume white microplate before the addition of 4 µL of the HTRF phospho-STAT6 (Tyr641) detection reagents. The HTRF signal was recorded after an overnight incubation.

STAT6 phosphorylation induced by IFNalpha dose-response
THP1 cells were seeded at different cell densities in a 96-well microplate, then stimulated with increasing concentrations of hIFNa for 20 minutes. Following the 2-plate assay protocol, 16 µL of lysate were transferred into a 384-well low volume white microplate before the addition of 4 µL of the HTRF phospho-STAT6 (Tyr641) detection reagents. The HTRF signal was recorded after an overnight incubation.

HTRF phospho-STAT6 cellular assay compared to Western Blot
The human Hela line was seeded in a T175 flask, and incubated at 37°C, 5% CO2, until confluency. The cells were then treated with IL4 (50 ng/mL) for 20 min before lysis. Serial dilutions of the cell lysate were performed in the supplemented lysis buffer, and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of HTRF phospho-STAT6 detection reagents. Equal amounts of lysates were used for a side by side comparison between HTRF and Western Blot. Using the HTRF Phospho-STAT6 Y641 assay, 5,000 cells/well were sufficient to detect a signal, while 40,000 cells were needed using Western Blot with an ECL detection. These results demonstrate that the HTRF phospho-STAT6 assay is 8 times more sensitive than the Western Blot

Simplified pathway
Function and regulation of STAT6
STAT6 is phosphorylated in response to cytokines and growth factors by the receptor associated kinase, JAK. Once phosphorylated, STAT6 proteins form homo or heterodimers and translocate into the nucleus, where they mediate cytokine induced gene expression. Interleukin 4 is the main cytokine triggering STAT6 phosphorylation on tyrosine 641 residue and inducing the expression of BCL2L1/BCL-X(L), which is responsible for the anti-apoptotic activity of IL4. To a lesser extent, STAT6 phosphorylation by TBK1 has been shown to be associated with the STING pathway, thus playing a role in innate immunity in response to viral infection.

Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 3
Lysis Buffer 4
Lysis Buffer 5
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Infectious Diseases
Oncology & Inflammation
|
| Unit Size |
500 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
Atherosclerosis pathogenesis, cellular actors, and pathways
Atherosclerosis is a common condition in which arteries harden and...
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.
Advance your autoimmune disease research and benefit from Revvity broad offering of reagent technologies


Loading...
How can we help you?
We are here to answer your questions.