

HTRF SARS-CoV2 Nucleocapsid Detection Kit, 500 Assay Points





| Feature | Specification |
|---|---|
| Application | Protein Quantification |
| Dynamic Range | 307 - 75,000 pg/mL |
| Limit of Detection | 165 pg/mL |
| Sample Volume | 16 µL |









Product information
Overview
The SARS-CoV-2 N protein is the basal monomeric constituent of the coronavirus oligomeric nucleocapsid. This protein is major immunogen predominantly expressed at early stages of viral infection and present from day one in infected patients' serum. As such, SARS-CoV-2 N protein is a reliable marker for viral infection and a possible immunogen candidate for the development of a vaccine.
How it works
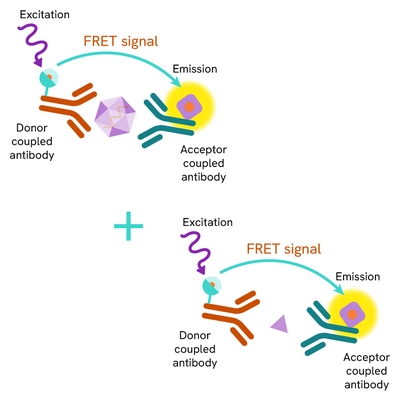
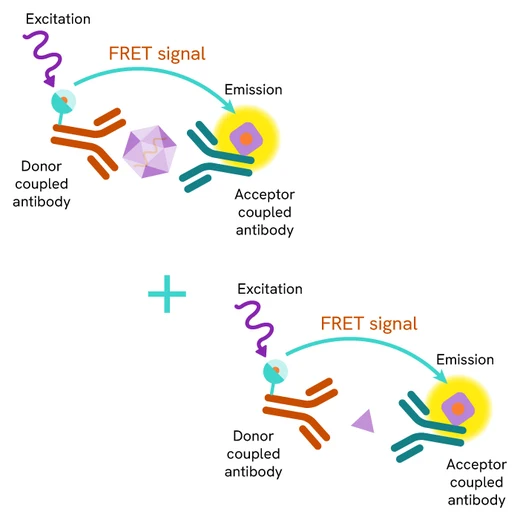
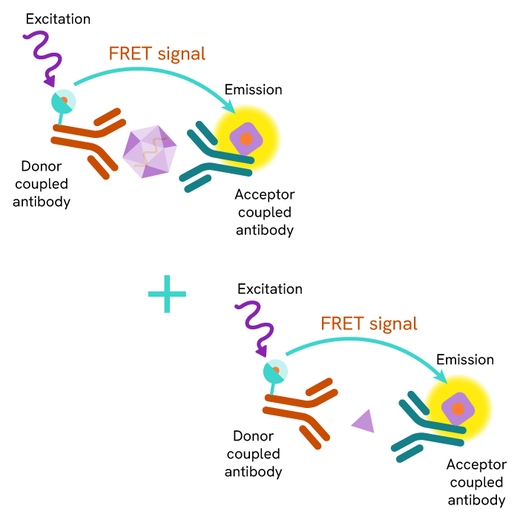
SARS-CoV-2 particles are detected in cell lysates using a sandwich immunoassay involving an anti-nucleocapsid monoclonal antibody, labelled with terbium-Cryptate or d2, ensuring assay quality, reproducibility, and signal quality. The specific HTRF signal generated is proportional to the amount of nucleocapsid proteins.

The assay protocol, using a 384-well small volume plate or a low volume 96 well plate (20 µL final), is described on the right. 16 µL of cell lysates or control are dispensed directly into the detection plate for detection by HTRF reagents. The antibodies labelled with HTRF donor and acceptor can be pre-mixed and added in a single dispensing step to further streamline the assay procedure (4 µL), or separately (2 µL).

Assay details
Detection of recombinant SARS-CoV-2 nucleocapsid
16 µL of recombinant protein in lysis buffer #5 were dispensed into a low volume white microplate before the addition of 4 µL of the premixed HTRF anti SARS-CoV2 capsid detection reagents. The HTRF signal was recorded after 2h incubation at RT.

Standard curve Capsid kit
| Sample size | 16 µL |
|---|---|
| Final assay volume | 20 µL |
| Time to result | 2h at RT |
| Detection limit | 165 pg/mL |
| Dynamic range | 307 to 75,000 pg/mL |
| Sample compatibility | Cell-based or cell supernatant |

Specifications
| Application |
Protein Quantification
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Dynamic Range |
307 - 75,000 pg/mL
|
| Limit of Detection |
165 pg/mL
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Viral Particles
|
| Technology |
TR-FRET
|
| Unit Size |
500 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.
Drug Repurposing can be an effective way to identify treatments for diseases, especially when time is of the essence.
Research...


Loading...
How can we help you?
We are here to answer your questions.






























