

HTRF Human C1q Binding Kit, 100 Assay Points





| Feature | Specification |
|---|---|
| Application | Protein-Protein Interaction |
| Sample Volume | 5 µL |









Product information
Overview
Complement-Dependent Cytotoxicity (CDC) is an important effector function of antibodies. Activation of the classical complement pathway is initiated by the binding of the complement protein C1q to the Fc region of immunoglobulins bound to a cell surface target antigen. This interaction triggers the sequential enzymatic activation of circulating complement proteins, and finally leads to the formation of the Membrane Attack Complex (MAC) that mediates target cell lysis.
The capacity of C1q binding and resultant CDC activity can influence the safety and efficacy of therapeutic monoclonal antibodies, and therefore requires characterization during the development process. Engineering the Fc region of IgG antibodies (e.g. by mutations or glycoengineering) is currently an active area of investigation to enhance or reduce the ability of biologics to recruit complement.
The human C1q binding kit measures the binding of the Fc region of aggregated IgG antibodies to human C1q in solution.
How it works
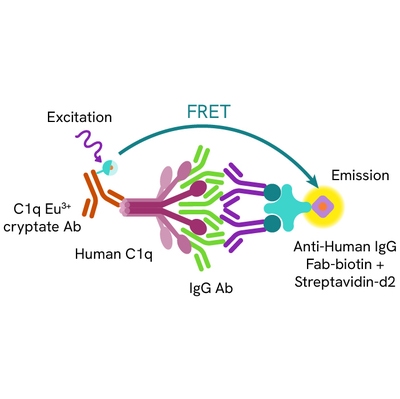
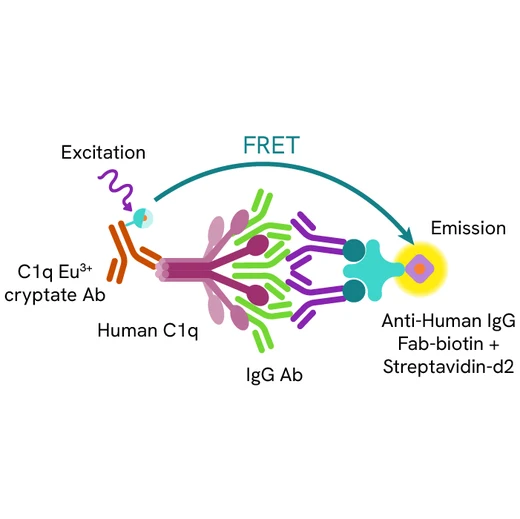
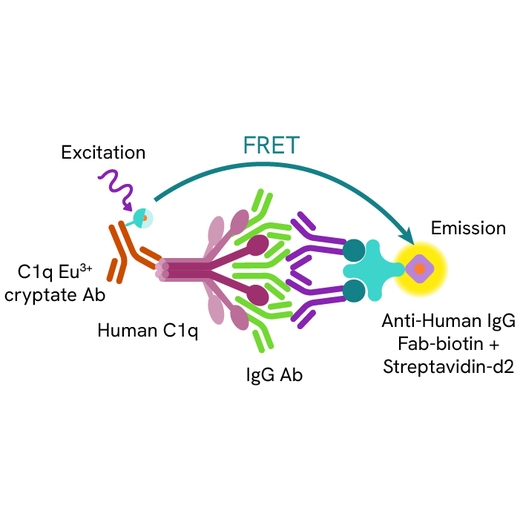
HTRF Human C1q Binding assay principle
The HTRF Human C1q binding assay measures the binding of the Fc region of IgG antibodies to human C1q in buffered solution. Unlike conventional methods that require the immobilization of antibodies on a solid phase, this assay enables simple and rapid characterization of antibodies in a homogeneous generic format. A biotinylated anti-human IgG Fab antibody complexed to streptavidin is used to capture and aggregate the tested antibody in solution. Antibodies bound to human C1q are detected in a sandwich assay format using an anti-C1q antibody labelled with Eu3+ cryptate (donor), and the streptavidin labelled with d2 (acceptor). The interaction between the two partners brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the capacity of the antibody Fc region to interact with human C1q.

HTRF Human C1q Binding assay protocol
The human C1q binding assay can be run in a 96- or 384-well low volume white plate (20 µL final). It requires only 1 incubation step after the sequential addition of assay reagents. As described here, tested antibodies (or the standard) are dispensed directly into the assay plate. The premix [Anti-Human IgG-Fab-biotin + Streptavidin-d2] is then added, followed by the dispensing of the human C1q protein and the anti-C1q antibody labelled with Eu3+ Cryptate. After a 3h-incubation at RT, the FRET signal can be recorded on an HTRF compatible reader. No washing steps are needed. The protocol can be further miniaturized or upscaled by simply resizing each addition volume proportionally.

Assay validation
Binding profiles of human IgG isotype controls
Purified human IgG1, IgG2 and IgG4 isotype control antibodies were serially diluted with Diluent #12 and tested in the Human C1q binding assay. The signal intensity obtained for each dose-response binding curve shows that human IgG1 binds efficiently to C1q, and human IgG2 interacts weakly with the complement component, whereas human IgG4 does not bind to the protein. These results are in accordance with the literature (Almagro et al., 2018, Progress and Challenges in the Design and Clinical Development of Antibodies for Cancer Therapy. Front. Immunol. 8:1751).

Binding profiles of Rituximab variants
The therapeutic anti-CD20 antibody Rituximab (chimeric IgG1) was tested in the Human C1q binding assay in parallel with the following Rituximab variants: non-fucosylated Rituximab (InvivoGen, #hcd20-mab13), non-glycosylated Rituximab (InvivoGen, #hcd20-mab12), Rituximab IgG2 isotype (InvivoGen, #hcd20-mab2), and Rituximab IgG4 isotype (InvivoGen, #hcd20-mab4). As expected, the absence of the fucose residue on the Fc domain of the non-fucosylated antibody (supposed to modulate ADCC activity only) did not induce any detectable change in C1q binding compared to Rituximab. Conversely, the capacity of interaction of the non-glycosylated variant was altered, demonstrating that antibody Fc glycosylation is essential for Fc-mediated effector mechanisms. Finally, isotype switching from IgG1 to IgG2 or IgG4 induced a considerable decrease or an elimination of the capacity of the antibody to bind to C1q.

Binding profiles of therapeutic type I & type II anti-CD20 antibodies
The therapeutic anti-CD20 antibodies Rituximab (type I, chimeric IgG1) and Obinutuzumab (type II, humanized IgG1, also known as 'GA101') were tested in the Human C1q binding assay. The lower signal obtained with the type II anti-CD20 Obinutuzumab demonstrates its reduced capacity to bind to C1q compared to the type I anti-CD20 Rituximab. These results are consistent with literature (Herter et al., 2013, Preclinical Activity of the Type II CD20 Antibody GA101 (Obinutuzumab) Compared with Rituximab and Ofatumumab In Vitro and in Xenograft Models, Mol Cancer Ther; 12(10); 2031–42).

Binding profiles of Cetuximab variants
The therapeutic anti-EGFR antibody Cetuximab (chimeric IgG1) was tested in the Human C1q binding assay in parallel with the following Cetuximab variants: non-glycosylated Cetuximab (InvivoGen, #hegfr-mab12) and Cetuximab IgG2 isotype (InvivoGen, #hegfr-mab2). As expected, the binding capacity of the non-glycosylated variant was altered, demonstrating that antibody Fc glycosylation is essential for Fc-mediated effector mechanisms. Isotype switching from IgG1 to IgG2 also induces a considerable decrease in the capacity of the antibody to interact with C1q.

Binding profiles of Ipilimumab and IgG2 isotype variant
The therapeutic anti-CTLA-4 antibody Ipilimumab (fully human IgG1) was tested in the Human C1q binding assay in parallel with its IgG2 isotype variant (InvivoGen, #hctla4-mab2). The signal decrease observed between the biologic and its variant demonstrates that the isotype switching from IgG1 to IgG2 leads to a considerable loss of capacity of the antibody to bind to C1q.

Binding profiles of therapeutic anti-PD-L1 & anti-PD-1 antibodies
The therapeutic antibodies Atezolizumab (anti-PD-L1, humanized IgG1) and Spartalizumab (anti-PD-1, humanized IgG4) were tested in the Human C1q binding assay. As expected, the anti-PD-L1 IgG1 antibody Atezolizumab showed strong binding to C1q, whereas the anti-PD-1 IgG4 antibody Spartalizumab did not have the capacity to interact with C1q.

Binding profiles of therapeutic anti-TNF-α antibodies
The therapeutic anti-TNF-α antibodies Infliximab (chimeric IgG1) and Adalimumab (fully human IgG1) were tested in the Human C1q binding assay. The binding dose-response curves showed similar profiles and signal intensity, demonstrating that both anti-TNF-α antibodies have the same capacity to interact with C1q.

Specifications
| Application |
Protein-Protein Interaction
|
|---|---|
| Automation Compatible |
Yes
|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Product Group |
Kit
|
| Sample Volume |
5 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Binding Assay
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Inflammation
Oncology & Inflammation
|
| Unit Size |
100 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
A comprehensive overview of the autoimmunity world
This guide provides information on the diversity of the cell types and molecular...
Over the last decade, therapeutic antibodies have emerged as the predominant class of new drugs. Assays to functionally...
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.


Loading...
How can we help you?
We are here to answer your questions.






























