
HTRF Human and Mouse Total IKZF1 Detection Kit, 500 Assay Points
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |



Product information
Overview
The Ikaros zinc-finger (IKZF) family of transcription factors are essential regulators of lymphopoiesis. IKZF1 (Ikaros) and IKZF3 (Aiolos) function as both transcriptional repressors and activators during T and B cell differentiation, as well as in the function of mature cells. Ikaros family proteins partner with multiple complexes, including NuRD, PRC2, and transcription elongation factors. Through these associations, they can modulate gene expression profiles and the chromatin state. The overexpression of these proteins has been linked to sustaining cancer cell proliferation and survival, such as malignant plasma cells and multiple myeloma.
Among the therapeutic strategies investigated to fight cancers, the use of IMIDs (immunomodulatory imide drugs) and their derivatives, termed CELMoDs (Cereblon E3 Ligase Modulatory Drugs) has become a popular strategy. By binding to CRBN, these compounds induce the ubiquitination of targeted proteins and promote their subsequent degradation. CELMoDs targeting IKZF1 and IKZF3 have shown promising anti-tumor activity in multiple myeloma cell models.
How it works
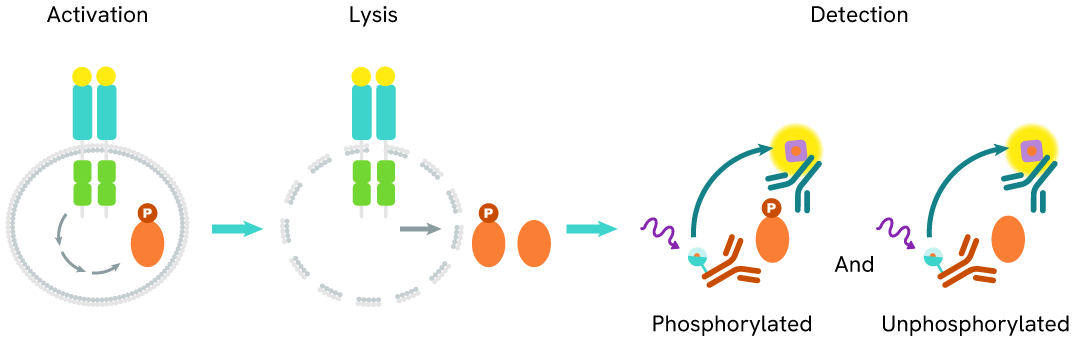
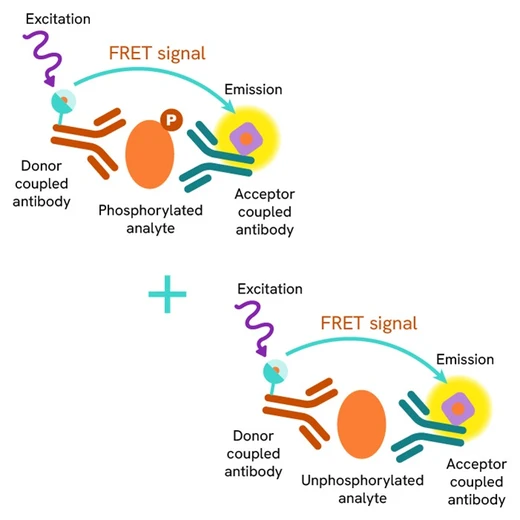
Total IKZF1 assay principle
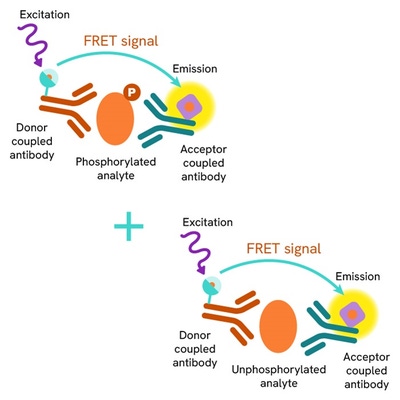
The HTRF Total IKZF1 assay quantifies the expression level of IKZF1 in a cell lysate. Unlike Western Blot, the assay is entirely plate-based, and does not require gels, electrophoresis, or transfer. The Total IKZF1 assay uses two labeled antibodies, one coupled to a donor fluorophore and the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In presence of IKZF1 in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor, and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample, and provides a means of assessing the protein’s expression under a no-wash assay format.

Total IKZF1 two-plate assay protocol
The 2-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of the Total IKZF1 HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.
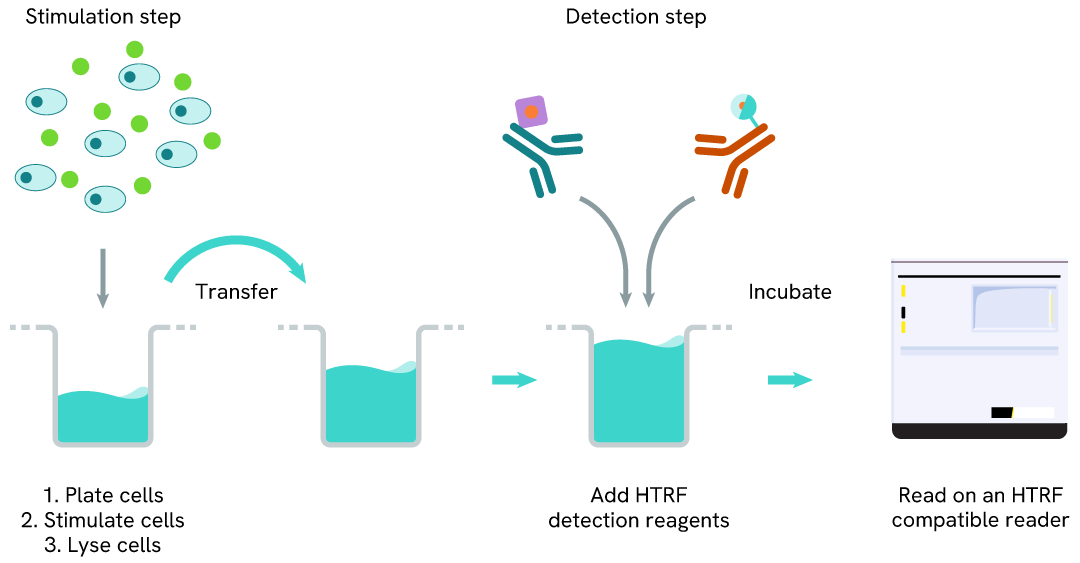
Total IKZF1 one-plate assay protocol
Detection of Total IKZF1 with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS-designed protocol enables miniaturization while maintaining robust HTRF quality.
Assay validation
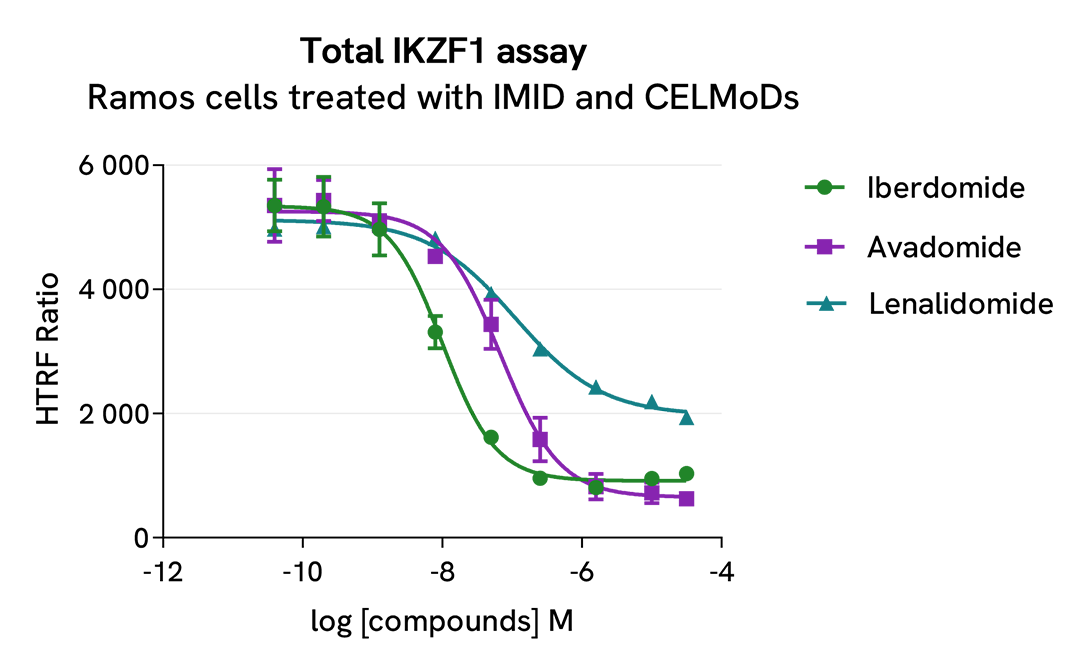
HTRF Total IKZF1 modulation using IMID and CELMoDs on Ramos cells
Ramos cells were seeded in 96-well culture plate (50,000 cells/30µL well) and treated with increasing concentrations of the IMID Lenalidomide or the CELMoDs Iberdomide and Avadomide, described as inducing IKZF1 and IKZF3 degradation.
After overnight incubation, cells were lysed with 10 µL of supplemented lysis buffer #4 (4X) for 30 min at RT under gentle shaking. Next, 16 µL of lysate were transferred into a 384-well low volume white microplate, and 4 µL of the HTRF Total IKZF1 detection antibodies were added. An additional 4 µL of lysate (supplemented with 12 µL diluent #8) were also transferred into the microplate to check the alpha-tubulin level with the alpha-tubulin housekeeping Cellular Kit (64ATUBPET/G/H). HTRF signals were recorded after an overnight incubation.
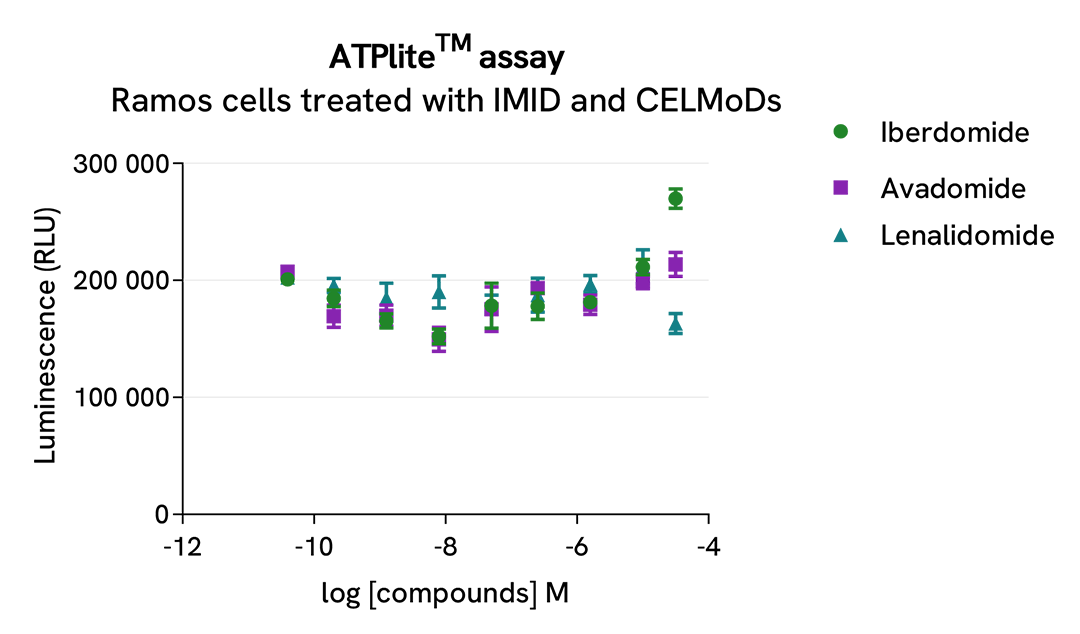
The three compounds triggered an IKZF1 dose-dependent decrease in protein expression level, whereas the alpha-tubulin expression level as well as ATP levels remained stable as expected.
Our results indicate that Iberdomide and Avadomide induce IKZF1 degradation with higher potency (DC50* 10nM and 64nM, respectively) and efficacy (81% and 88% degradation rate respectively) compared to Lenalidomide (DC50* 110nM and degradation rate 61%), as described in the literature by Fuchs et al. (Blood Reviews, 2023).
* DC50 corresponds to the concentration of the degrader at which 50% of the targeted protein is degraded.
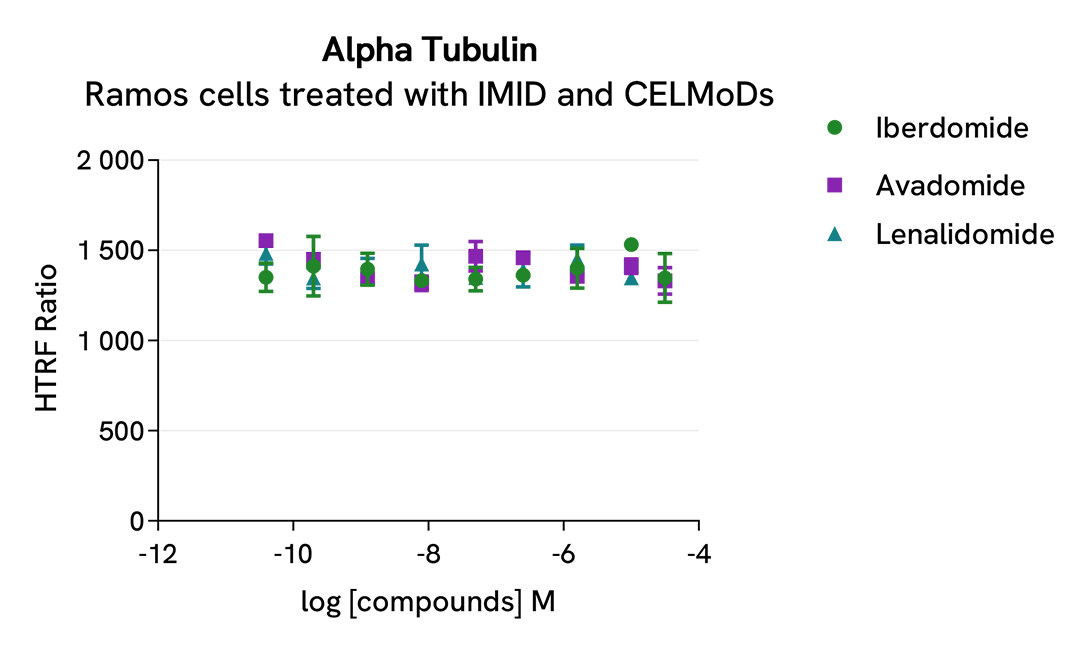
|
Molecular glue degrader |
Protein target |
E3 Ligase |
DC50 (nM) |
Degradation rate |
|---|---|---|---|---|
|
Lenalidomide (IMID) |
IKZF1 |
CRBN |
110 |
61% |
|
Iberdomide (CELMoD) |
IKZF1 |
10 |
81% |
|
|
Avadomide (CELMoD) |
IKZF1 |
64 |
88% |
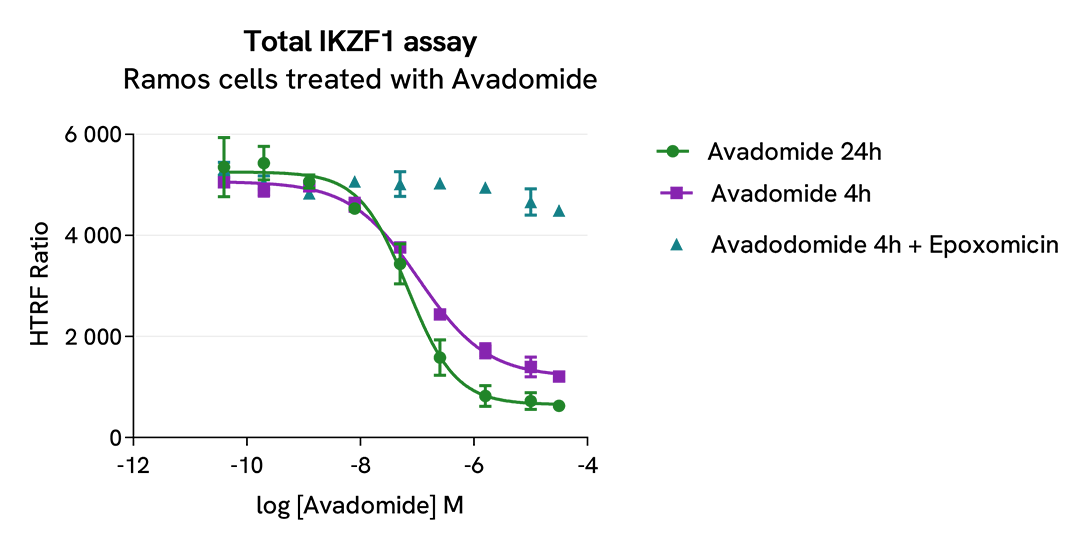
Total IKZF1 modulation using CELMoDs is mediated by ubiquitin-proteasome system
Ramos cells were seeded in 96-well culture plate (50,000 cells/30µL well) and treated with increasing concentrations of the CELMoD Avadomide for 4h or 24h. The proteasome inhibitor epoxomicin (1µM) was added or not 1h prior to the addition of Avadomide .
After treatment, cells were lysed with 10 µL of supplemented lysis buffer #4 (4X) for 30 min at RT under gentle shaking. Next, 16 µL of lysate were transferred into a 384-well low volume white microplate, and 4 µL of the HTRF Total IKZF1 detection antibodies were added. An additional 4 µL of lysate (supplemented with 12 µL diluent #8) were also transferred into the microplate to check the alpha-tubulin level with the alpha-tubulin housekeeping Cellular Kit (64ATUBPET/G/H). HTRF signals were recorded after an overnight incubation.
The CELMoD Avadomide induced a dose-dependent decrease in IKZF1 protein levels with DC50* of 107 nM and 64 nM after 4h and 24h, respectively. Furthermore, Avadomide-induced IKZF1 degradation was prevented in the presence of epoxomicin, a proteasome activity inhibitor. This result unambiguously demonstrates that Avadomide-induced IKZF1 degradation is mediated by the ubiquitin proteasome system. Similar data were obtained when treating cells with Lenalidomide (IMID) or Iberdomide (CELMoD) (data not shown).
* DC50 corresponds to the concentration of the degrader at which 50% of the targeted protein is degraded.
HTRF Total IKZF1 versus IKZF3 modulation using IMID and CELMoDs on Ramos cells
Ramos cells were seeded in 96-well culture plate (50,000 cells/30µL well) and treated with increasing concentrations of the IMID Lenalidomide or the CELMoDs Iberdomide and Avadomide, described as inducing IKZF1 and IKZF3 degradation.
After overnight incubation, cells were lysed with 10 µL of supplemented lysis buffer #4 (4X) for 30 min at RT under gentle shaking. Next, 16 µL of lysate were transferred into a 384-well low volume white microplate, and 4 µL of the Total IKZF1 or Total IKZF3 detection antibodies were added. HTRF signals were recorded after an overnight incubation.
The three compounds triggered an IKZF1 and IKZF3 dose-dependent decrease in protein expression, whereas the alpha-tubulin expression level as well as ATP levels remained stable as expected (data not shown). Our results indicate that Lenalidomide, Iberdomide and Avadomide are equally potent to induce either IKZF1 or IKZF3 degradation.
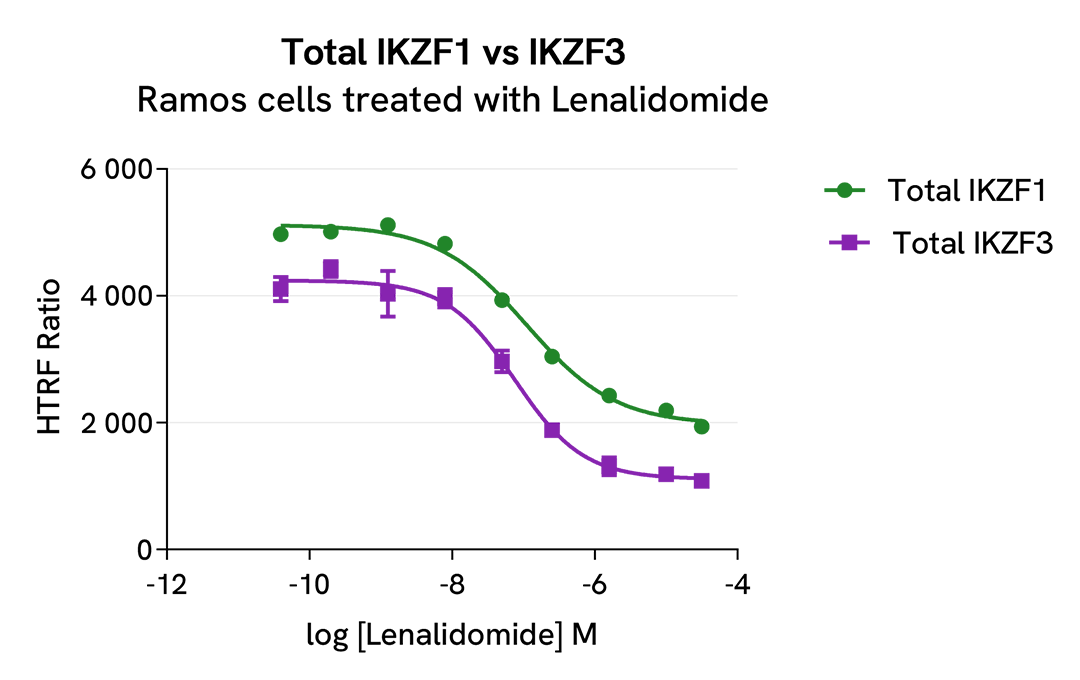
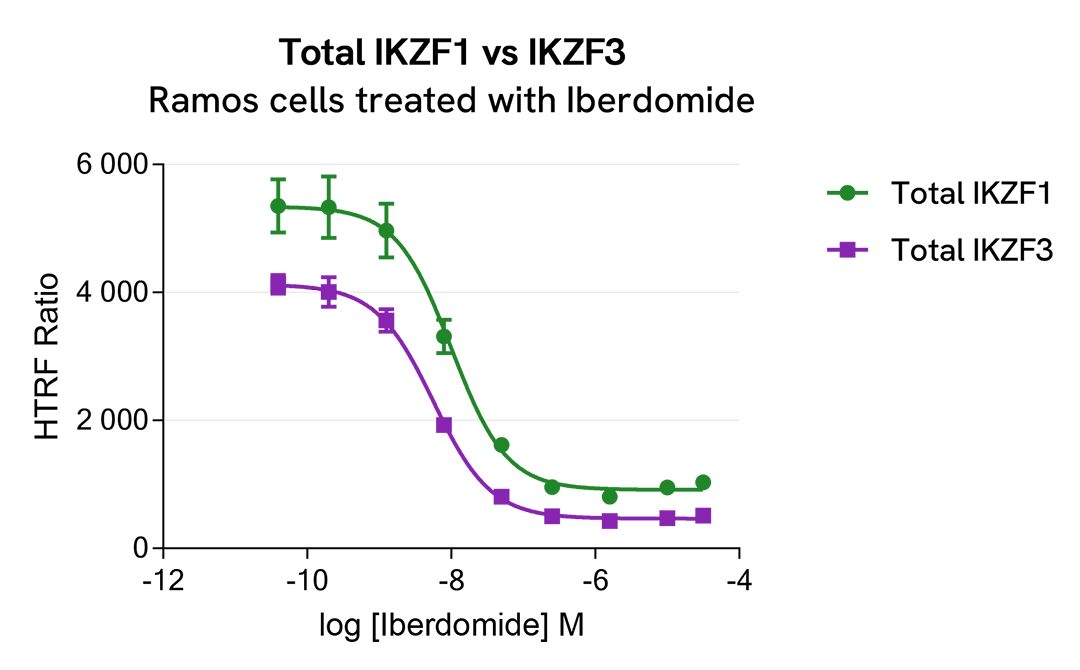
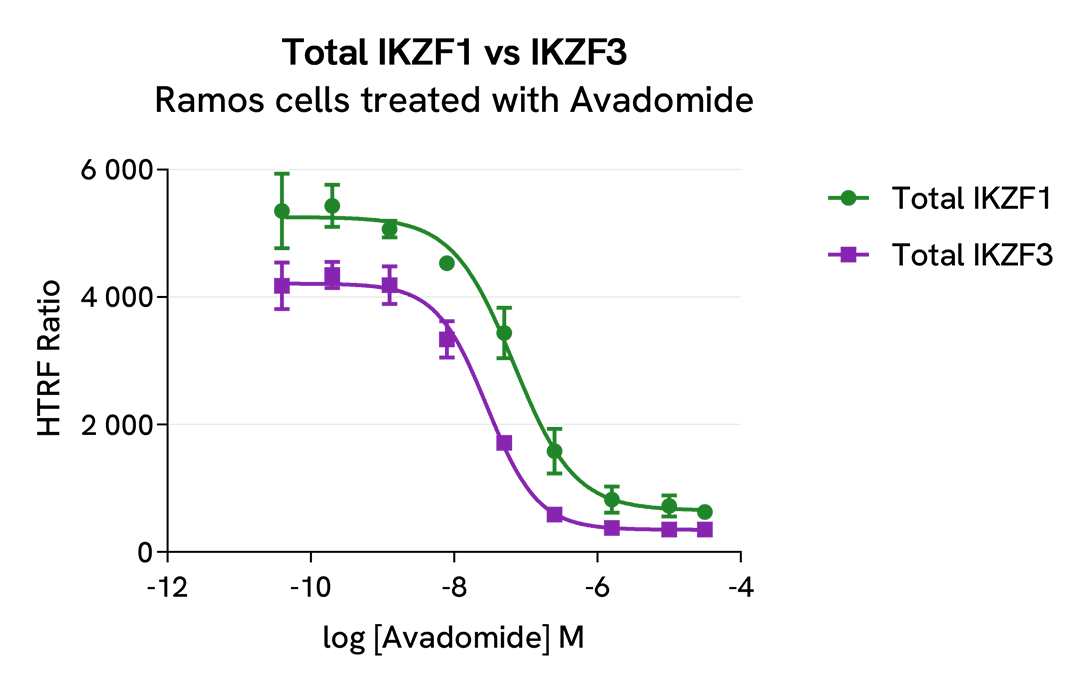
|
Molecular glue degrader |
E3 Ligase |
Protein target |
DC50 (nM) |
Degradation rate |
|---|---|---|---|---|
|
Lenalidomide (IMID) |
CRBN |
IKZF1 |
110 |
61% |
|
IKZF3 |
76 |
74% |
||
|
Iberdomide (CELMoD) |
IKZF1 |
10 |
81% |
|
|
IKZF3 |
6 |
88% |
||
|
Avadomide (CELMoD) |
IKZF1 |
64 |
88% |
|
|
IKZF3 |
29 |
92% |
Specificity and selectivity of HTRF Total IKZF1 assay
HAP1 cells were transfected with human IKZF1, human IKZF3 plasmids in a T175 flask and incubated for 24h. After medium removal, cells were lysed with 3 mL of supplemented lysis buffer #4 (1X) for 30 min at RT under gentle shaking.
The IKZF1 expression levels were assessed with the HTRF Total IKZF1 kit. Briefly, 16 µL of cell lysate were transferred into a low volume white microplate followed by 4 µL of premixed HTRF detection reagents. The HTRF signal was recorded after an overnight incubation at RT.
The HTRF Total IKZF1 assay efficiently detected IKZF1 in the IKZF1 transfected HAP1 cells compared to the WT HAP1 cells. No signal was detected in IKZF3 transfected cells, demonstrating the specificity and selectivity of the HTRF Total IKZF1 kit.
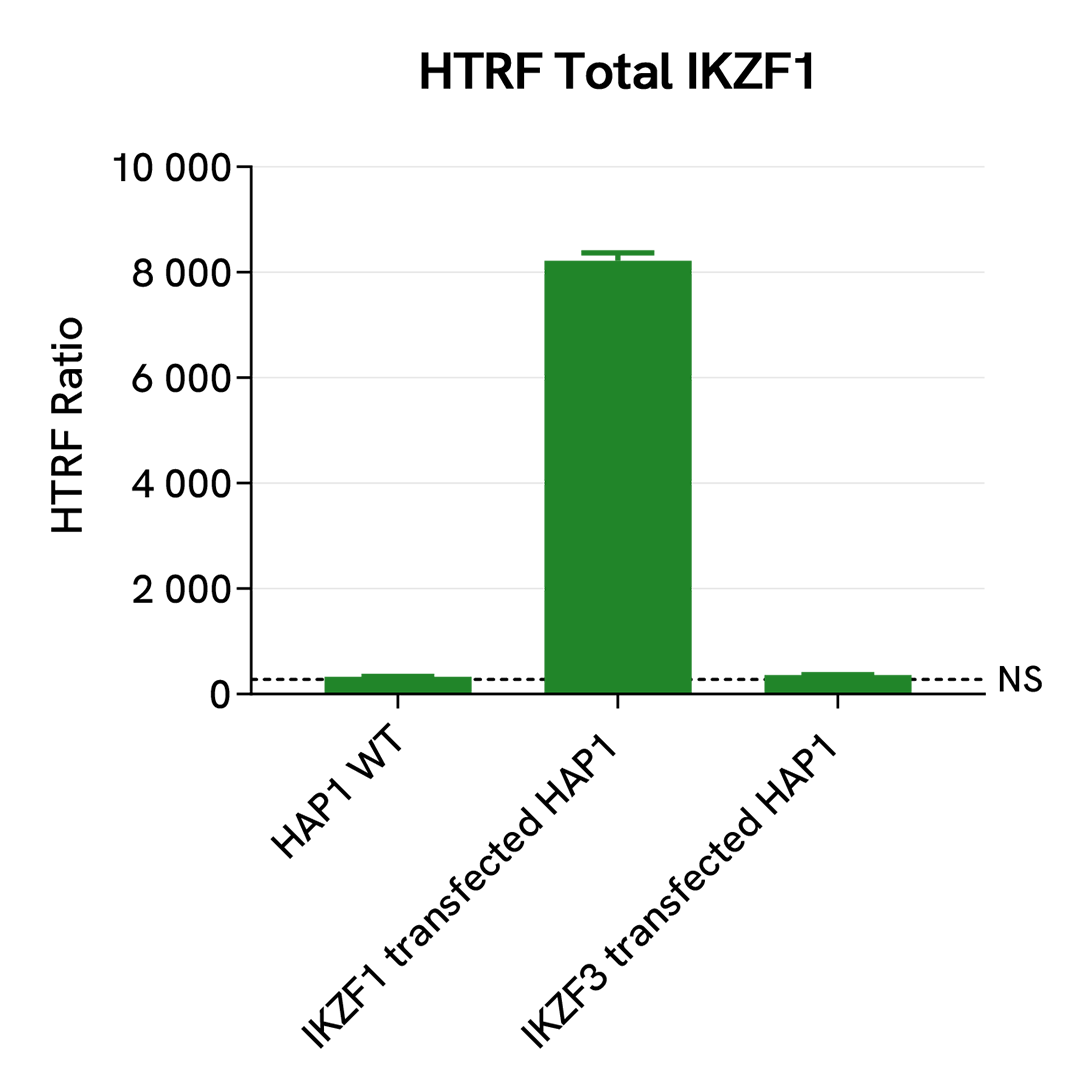
Validation on various Human and Mouse cell lines
The suspension cell lines MOLT-4 (T lymphoblast), Jurkat (T lymphocyte), THP-1 (acute monocytic leukemia), MV-4-11(B-myelomonocytic leukemia), Ramos (B lymphocyte), and Raji (B lymphoblastoid) were dispensed into a 96-well plate and lysed with 10 µL of supplemented lysis buffer #4 (4X) for 30 min at RT under gentle shaking (performed according to the suspension cell protocol).
The adherent cell line RAW 264.7 (murine macrophage) was plated in 96-well culture plate and incubated for 24 hours at 37°C, 5% CO2. After medium removal, cells were lysed with 50 µL of supplemented lysis buffer #4 (1X) for 30 min at RT under gentle shaking.
The IKZF1 expression level was assessed with the HTRF Total IKZF1 kit. Briefly, 16 µL of cell lysate were transferred into a low volume white microplate, followed by 4 µL of premixed HTRF detection reagents. The HTRF signal was recorded after an overnight incubation at RT. The dotted line corresponds to the non-specific HTRF signal. Note that the cell density was optimized beforehand to ensure HTRF detection within the dynamic range of the kit (data are shown for 100,000 cells/well)
The HTRF Total IKZF1 assay efficiently detected endogenous IKZF1 in various cellular models expressing different levels of the protein.
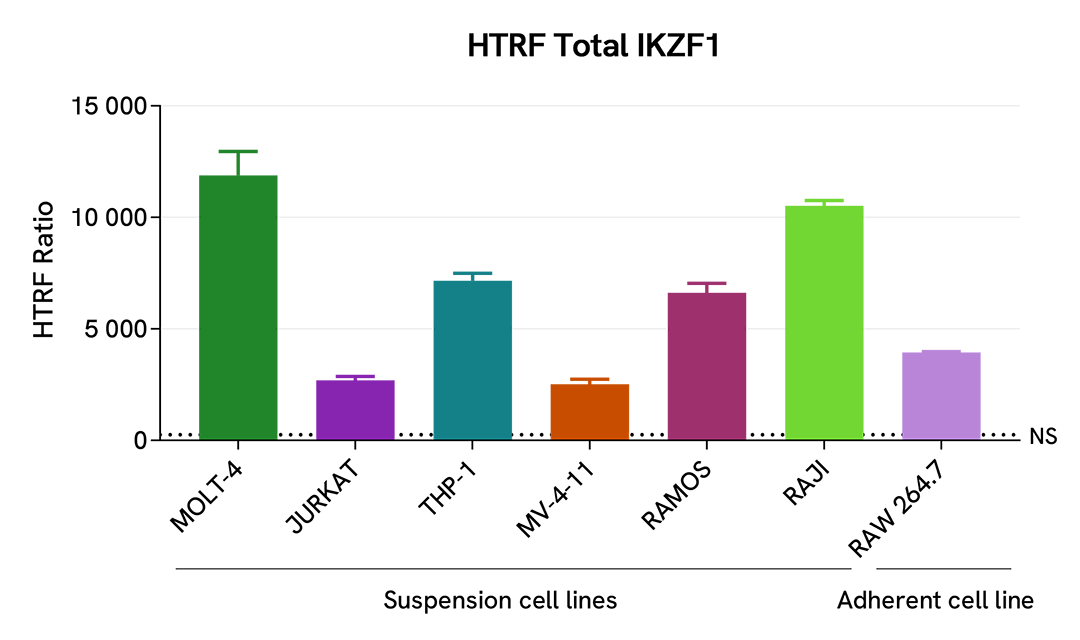
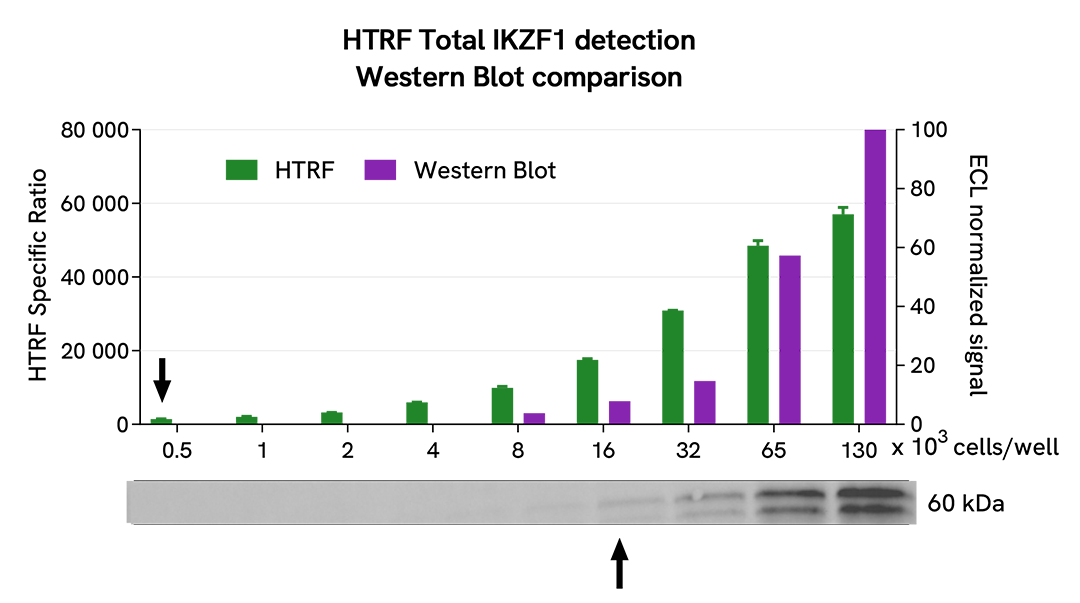
Comparison between HTRF and Western Blot sensitivity for Total IKZF1
Ramos cells were cultured in a T175 flask in complete medium at 37°C, 5% CO2. After medium removal, the cells were lysed with 3 mL of supplemented lysis buffer #4 (1X) for 30 min at RT under gentle shaking.
Serial dilutions of the cell lysate were performed using supplemented lysis buffer #4 (1X), and 16µL of each dilution were transferred into a 384-well small volume microplate before the addition of 4µL of HTRF Total IKZF1 detection antibodies. HTRF signals were recorded after an overnight incubation.
Equal amounts of lysates were loaded into a gel for a side-by-side comparison between HTRF and Western Blot.
In these conditions, the HTRF Total IKZF1 assay is 32-fold more sensitive than the Western Blot technique.
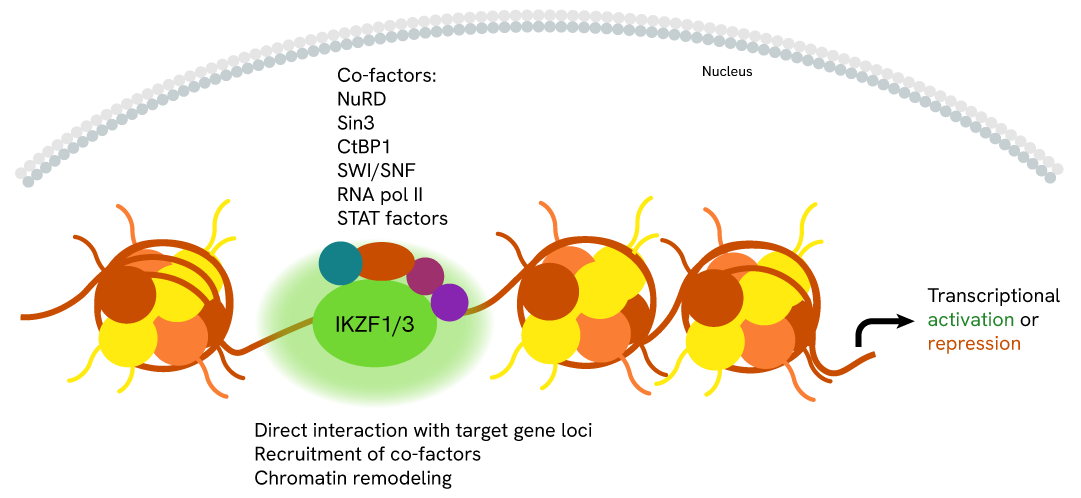
Simplified pathway
IKZF1 Signaling Pathway
Ikaros zinc finger transcription factors are important regulators of the gene programs underlying the development of hematopoietic cell lineages. They recruit different co-factors and interact with target gene loci or chromatin-remodeling complexes. Ikaros mediate different transcriptional outcomes by acting as transcriptional repressors or activators based on cell type.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Technology |
TR-FRET
|
| Unit Size |
500 assay points
|
Resources
Are you looking for resources, click on the resource type to explore further.
HTRF: unfamiliar territory?
This technical brochure reviews the general principles of HTRF™ and the associated Tag-lite™ technology...
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This application note compares six CRBN modulators using no-wash HTRF assays to quantify degradation across key neosubstrates. The...
Your guide for improving cell signaling assay performance
This complete Revvity guide provides you with all the tools you need to...


Loading...
How can we help you?
We are here to answer your questions.