
HTRF Human and Mouse Total STAT5 Detection Kit, 500 Assay Points
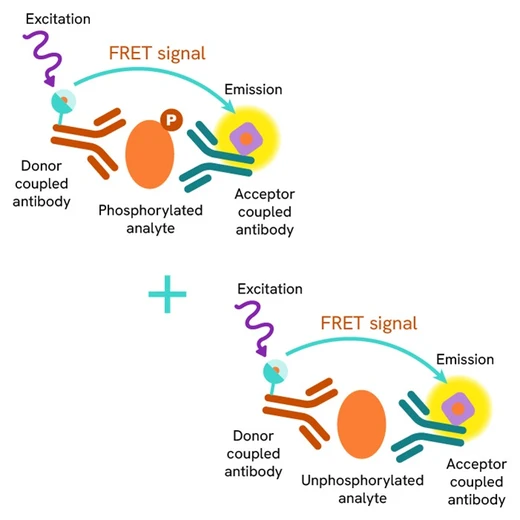
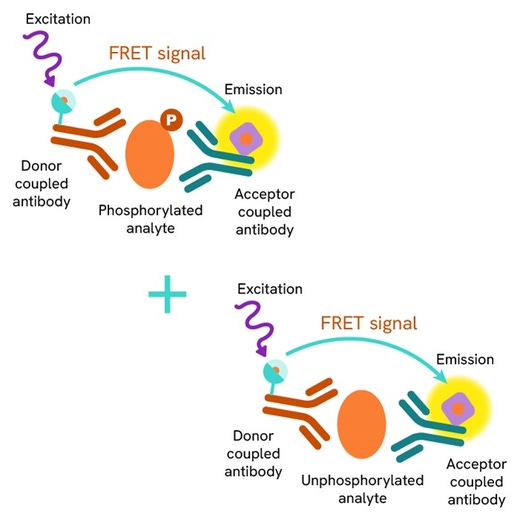
This HTRF kit allows for the cell-based quantitative detection of Total STAT5.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
This HTRF kit allows for the cell-based quantitative detection of Total STAT5.



Product information
Overview
Signal Transducer and Activator of Transcription (STAT) proteins are a seven-member family of cytoplasmic transcription factors that relay signals emanating from cell-surface cytokine and growth factor receptors to the nucleus. STAT proteins control fundamental cellular processes, including cell survival, proliferation, differentiation, and immune responses. Dysregulation of STAT5 signaling is implicated in hematopoietic cancers, where it promotes cell survival, proliferation, and metastasis.
HTRF assays offer many advantages over other technologies:
- Homogeneous add-and-read format
- No wash steps
- Low background
- Straightforward miniaturization from 96- or 384-well microplates to high density assay formats such as 384-well low volume and 1536-well plates
- Stable signal, providing flexibility in time of readout or size of assays
How it works
Total STAT5 assay principle
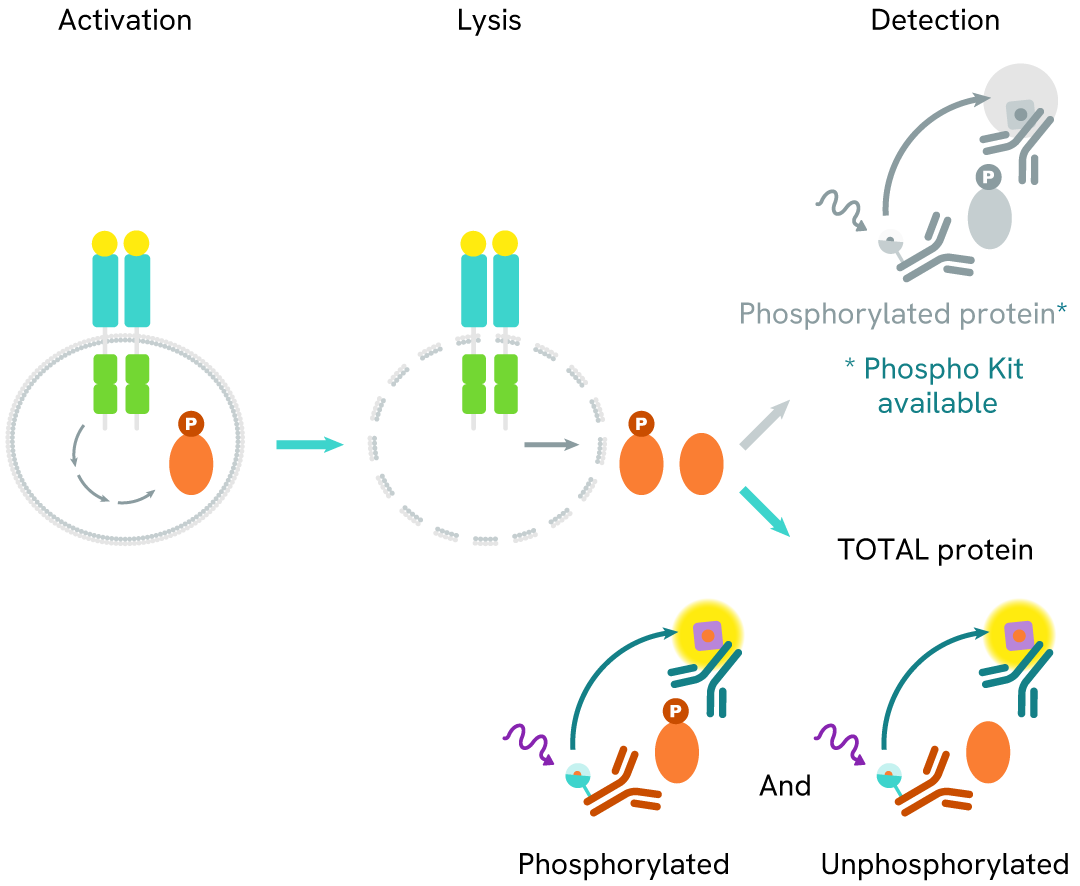
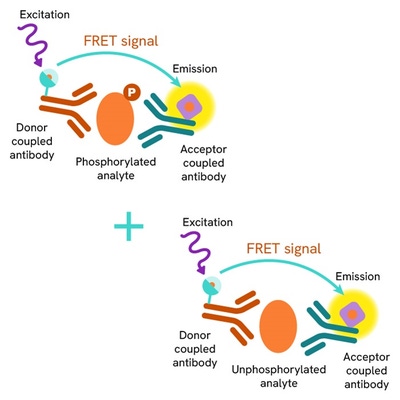
The Total STAT5 assay quantifies the expression level of STAT5 in a cell lysate. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The Total STAT5 assay uses two labeled antibodies, one coupled to a donor fluorophore and the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In the presence of STAT5 in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample and provides a means of assessing the protein's expression under a no-wash assay format.

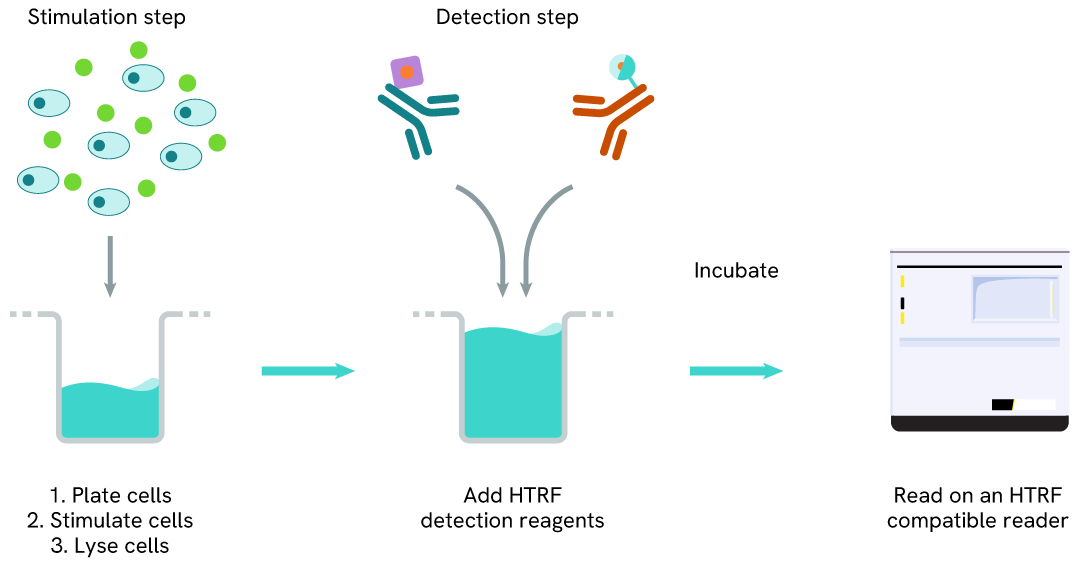
Total STAT5 two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of HTRF Total STAT5 detection reagents. This protocol allows for the cells' viability and confluence to be monitored.
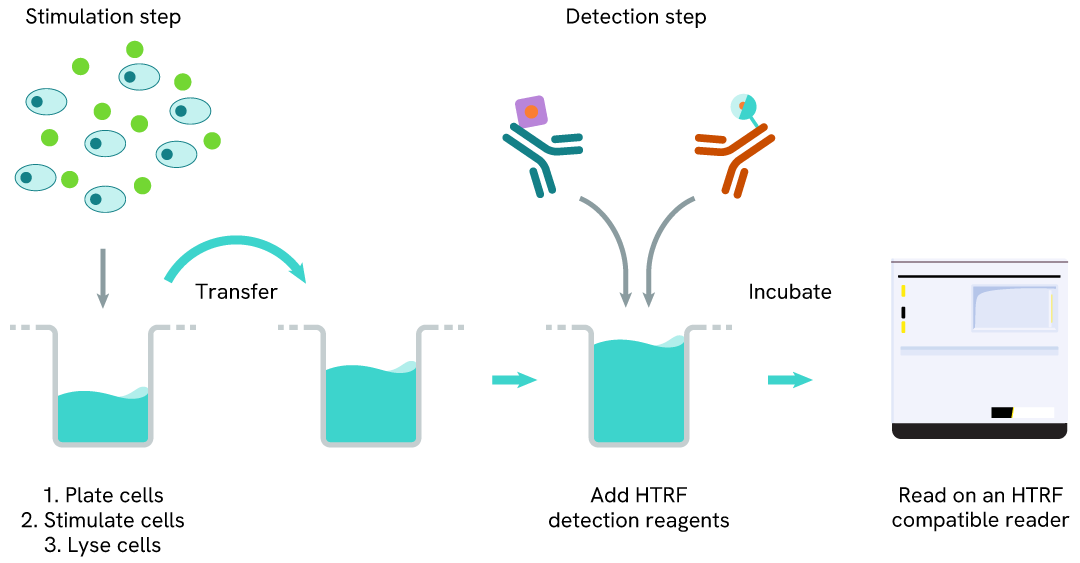
Total STAT5 one-plate assay protocol
Detection of Total STAT5 with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol facilitates miniaturization while maintaining robust HTRF quality.
Assay validation
PROTAC mediated STAT5 degradation in MOLT-4 cells
MOLT-4 cells were dispensed into a 96-well culture plate at a density of 200,000 cells per well and treated with increasing concentrations of the PROTAC AK-2292, known to induce Total STAT5 degradation.
Following overnight incubation, cells were lysed with 10 µL of supplemented lysis buffer #4 (4X) for 30 minutes at room temperature under gentle shaking. Subsequently, 16 µL of lysate was transferred into a 384-well low-volume white microplate, and 4 µL of HTRF total STAT5 detection antibodies were added. Additionally, 4 µL of lysate, supplemented with 12 µL of diluent #8, were transferred into the microplate to assess alpha-tubulin levels using the alpha-tubulin housekeeping Cellular Kit (64ATUBPET/G/H). HTRF signals were recorded after an overnight incubation.
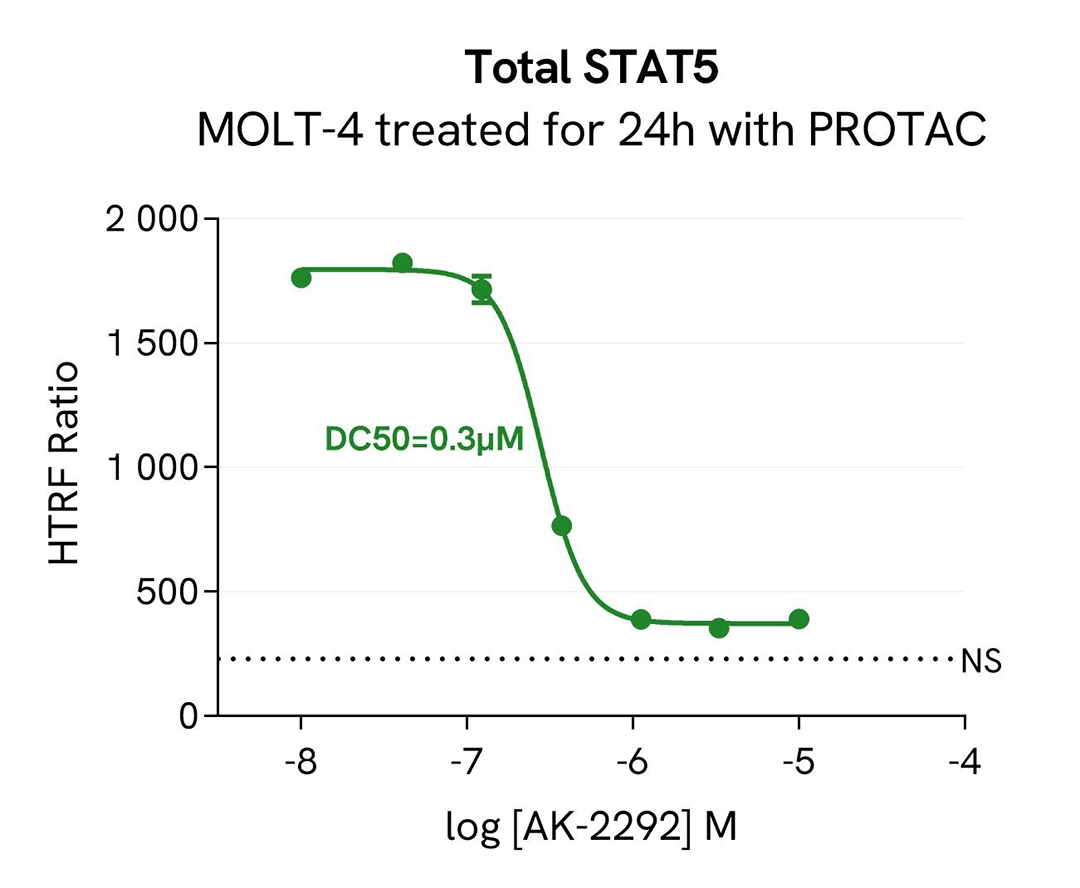
The AK-2292 PROTAC triggered a dose-dependent decrease in Total STAT5 protein expression level, while the alpha-tubulin expression level remained stable, as expected.
Our results indicate that AK-2292 induces Total STAT5 degradation with high potency (DC50* 0.3µM) and efficacy (80% degradation rate), as described in the literature by Kaneshige et al. (J. Med. Chem., 2023).
* DC50 corresponds to the concentration of the degrader at which 50% of the targeted protein is degraded.
HTRF Phospho-STAT5 (Tyr694) / Total STAT5 modulation using IFN- α on HeLa cells
HeLa cells were plated in a 96-well culture-treated plate at a density of 200,000 cells per well and allowed to adhere in complete culture medium for 24 hours. Subsequently, the cells were exposed to increasing concentrations of IFN-α for 30 minutes at 37°C, 5% CO2, in serum-free cell culture conditions. Following treatment, the cell culture medium was aspirated, and 50 µl of supplemented Lysis Buffer #4 (1X) was added to each well, followed by a 30-minute incubation at room temperature with gentle shaking.
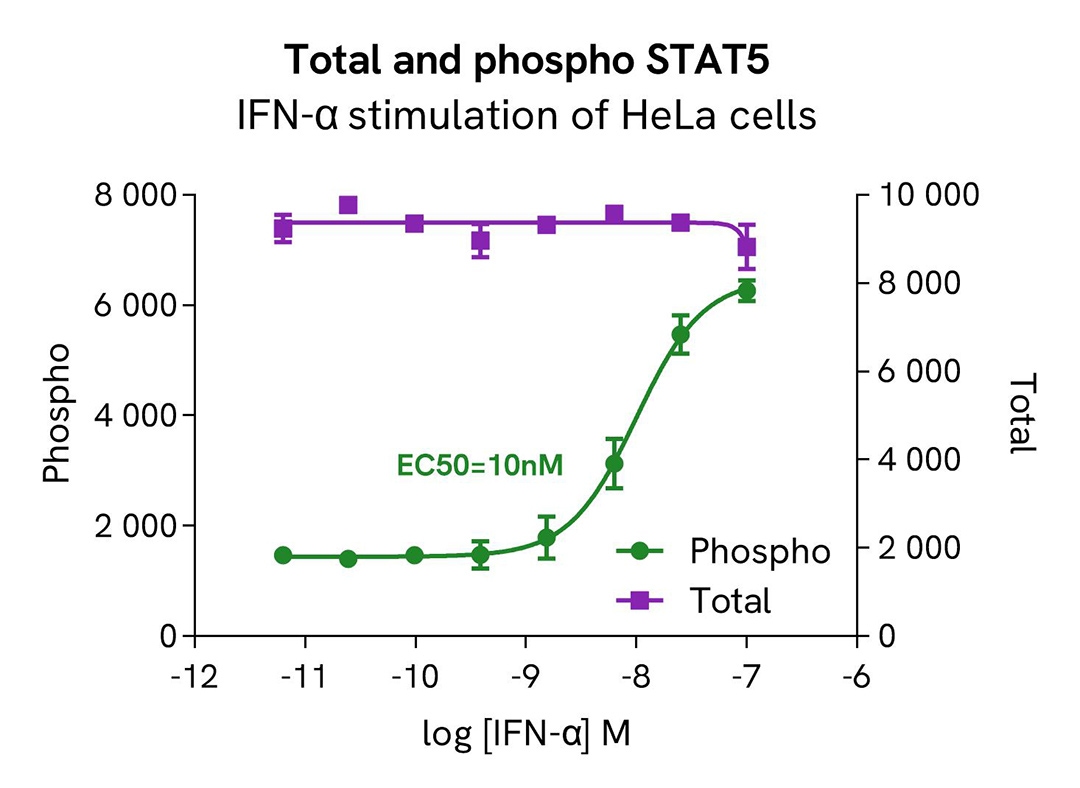
After cell lysis, 16 µL of lysates were transferred into a 384-well low-volume white microplate, and 4 µL of HTRF Total STAT5 or Phospho-STAT5 (Tyr694) (64AT5PEG/H) detection antibodies were added. An additional 4 µL of lysate (supplemented with 12 µL of diluent #8) were also transferred into the microplate to assess the alpha-tubulin level using the alpha-tubulin housekeeping Cellular Kit (64ATUBPET/G/H). HTRF signals were recorded after an overnight incubation.
The results demonstrated a dose-dependent activation of STAT5 phosphorylation at Tyr694 following treatment with IFN-α, while the total STAT5 protein expression level remained constant, as expected.
HTRF Phospho-STAT5 (Tyr694) / Total STAT5 modulation using IL-3 on TF-1 cells
TF-1 cells were plated in a 96-well culture plate at a density of 150,000 cells in 25 µL of medium per well and starved in serum-free medium for 2 hours at 37°C, 5% CO2. Subsequently, the cells were exposed to increasing concentrations of IL-3 for 10 minutes in serum-free cell culture conditions.
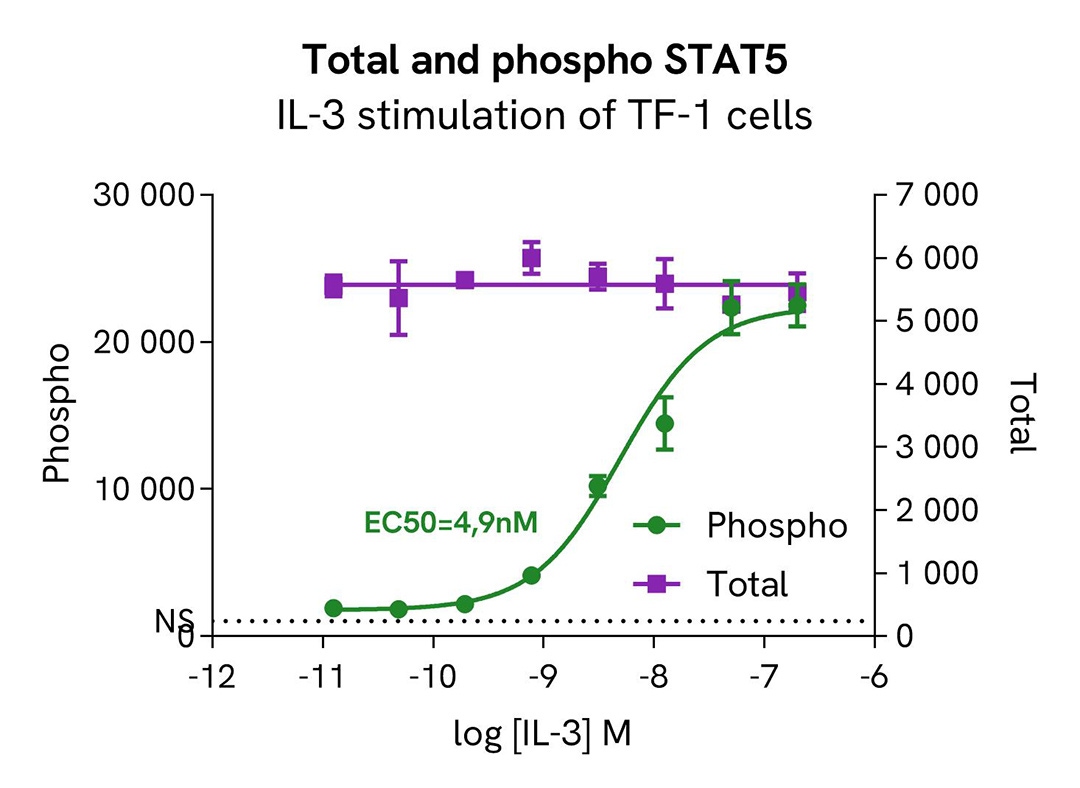
After treatment, the cells were lysed with 10 µL of supplemented lysis buffer #4 (4X) for 30 minutes at room temperature with gentle shaking. Then, 16 µL of lysates were transferred into a 384-well low-volume white microplate, and 4 µL of HTRF Total STAT5 or Phospho-STAT5 (Tyr694) (64AT5PEG/H) detection antibodies were added. An additional 4 µL of lysate (supplemented with 12 µL of diluent #8) were also transferred into the microplate to assess the alpha-tubulin level using the alpha-tubulin housekeeping Cellular Kit (64ATUBPET/G/H). HTRF signals were recorded after an overnight incubation.
Our results indicate a clear dose-dependent activation of STAT5 phosphorylation at Tyr694 upon treatment with IL-3, while the total STAT5 protein expression level remains constant.
HTRF Phospho-STAT5 (Tyr694) / Total STAT5 modulation using Lestaurtinib on HeLa cells
HeLa cells were plated in a 96-well culture-treated plate at a density of 200,000 cells per well and incubated for 24 hours in complete culture medium. They were then treated with increasing concentrations of the JAK inhibitor Lestaurtinib for 2 hours, followed by stimulation with 20nM IFN-α for 15 minutes at 37°C, 5% CO2, in serum-free culture medium.
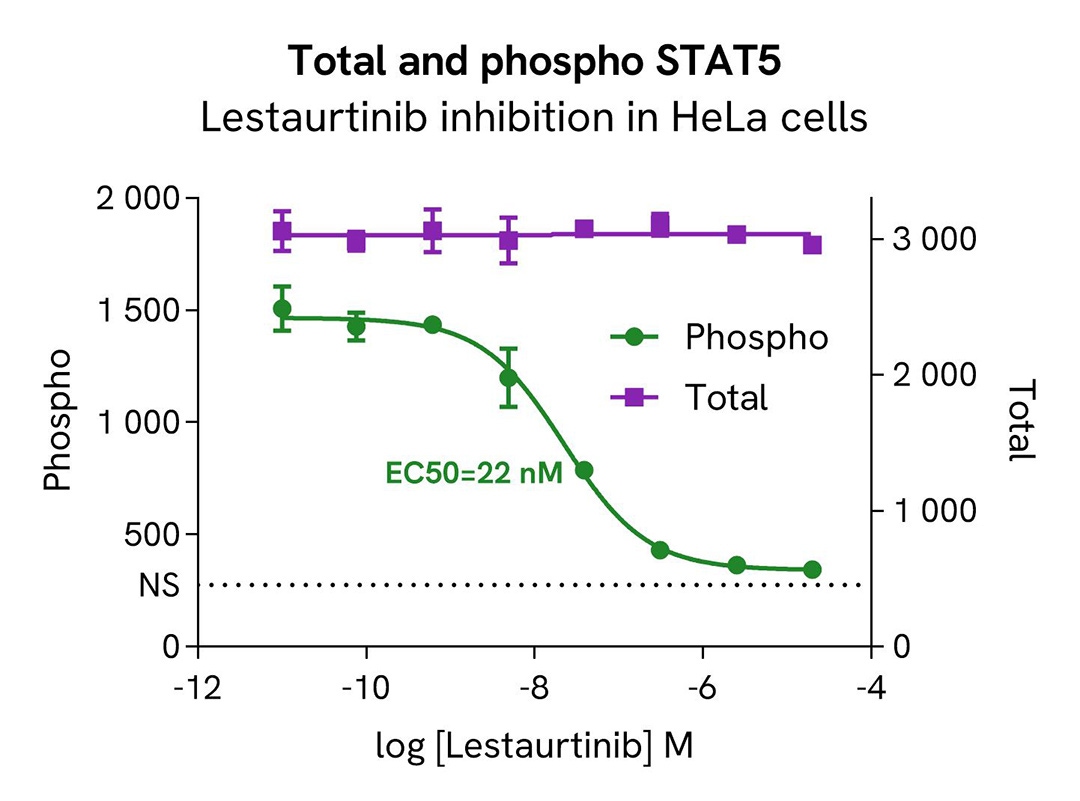
Post-treatment, the cell culture medium was aspirated, and 50 µl of supplemented Lysis Buffer #4 (1X) was added to each well, followed by a 30-minute incubation at room temperature with gentle shaking. Subsequently, 16 µL of lysates were transferred into a 384-well low-volume white microplate. 4 µL of HTRF Total STAT5 or Phospho-STAT5 (Tyr694) (64AT5PEG/H) detection antibodies were added to assess the levels of these proteins. Additionally, 4 µL of lysate, supplemented with 12 µL of diluent #8, were transferred into the microplate to measure alpha-tubulin levels using the alpha-tubulin housekeeping Cellular Kit (64ATUBPET/G/H). HTRF signals were recorded after an overnight incubation.
The results indicate a clear dose-dependent inhibition of STAT5 phosphorylation at Tyr694 upon treatment with the JAK inhibitor Lestaurtinib, while the total STAT5 protein expression level remains constant.
HTRF Phospho-STAT5 (Tyr694) / Total STAT5 modulation using Lestaurtinib on TF-1 cells
TF-1 cells were plated in a 96-well culture plate at a density of 200,000 cells in 20 µL of medium per well and starved in serum-free medium for 2 hours at 37°C, 5% CO2. Following starvation, the cells were treated with increasing concentrations of the JAK inhibitor Lestaurtinib for 2 hours, followed by stimulation with 50nM IL-3 for 10 minutes at 37°C, 5% CO2, in serum-free culture medium.
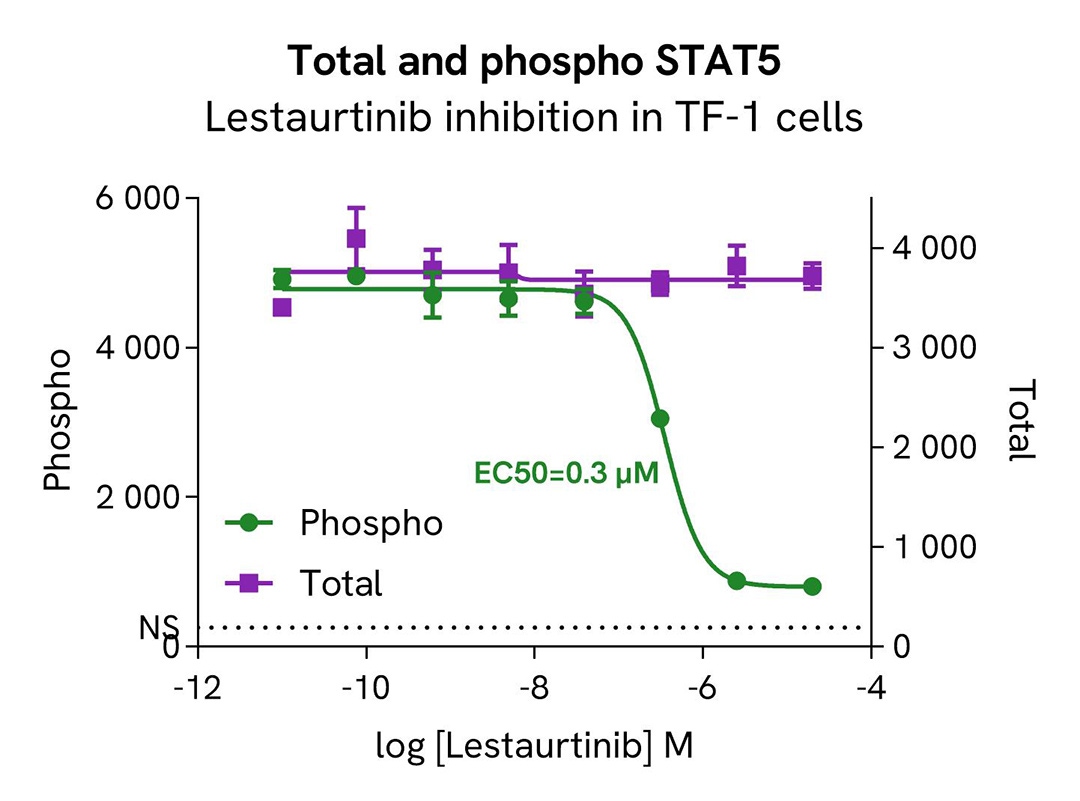
After treatment, the cells were lysed with 10 µL of supplemented lysis buffer #4 (4X) for 30 minutes at room temperature under gentle shaking. Subsequently, 16 µL of lysates were transferred into a 384-well low-volume white microplate. 4 µL of HTRF Total STAT5 or Phospho-STAT5 (Tyr694) (64AT5PEG/H) detection antibodies were added to assess the levels of these proteins. Additionally, 4 µL of lysate, supplemented with 12 µL of diluent #8, were transferred into the microplate to measure alpha-tubulin levels using the alpha-tubulin housekeeping Cellular Kit (64ATUBPET/G/H). HTRF signals were recorded after an overnight incubation.
Our results indicate a clear dose-dependent activation of STAT5 phosphorylation at Tyr694 upon treatment with the JAK inhibitor Lestaurtinib, while the total STAT5 protein expression level remains constant.
STAT5 expression level quantified in various cell lines
The suspension cell lines MOLT-4 (T lymphoblast) and Jurkat (T lymphocyte) were dispensed into a 96-well plate and lysed with 10 µL of supplemented lysis buffer #4 (4X) for 30 min at RT under gentle shaking (performed according to the suspension cell protocol).
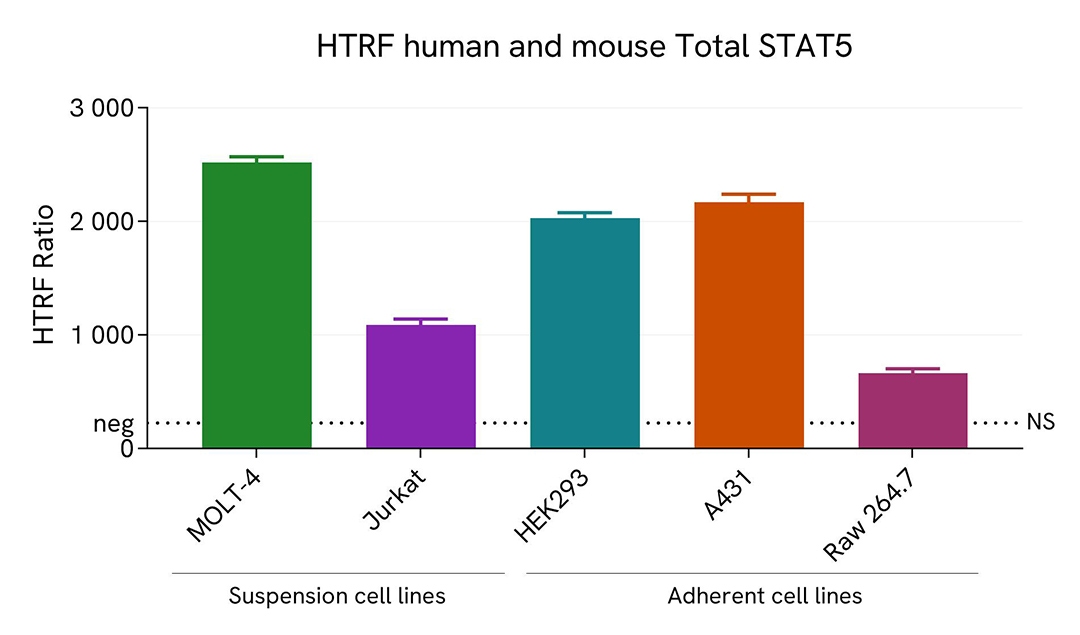
The adherent cell line HEK293 (human embryonic kidney), A431 (human epidermoid carcinoma) and RAW 264.7 (murine macrophage) were plated in 96-well culture plate and incubated for 24 hours at 37°C, 5% CO2. After medium removal, cells were lysed with 50 µL of supplemented lysis buffer #4 (1X) for 30 min at RT under gentle shaking.
The STAT5 expression level was assessed with the HTRF Total STAT5 kit. Briefly, 16 µL of cell lysate were transferred into a low volume white microplate, followed by 4 µL of premixed HTRF detection reagents. The HTRF signal was recorded after an overnight incubation at RT. The dotted line corresponds to the non-specific HTRF signal. Note that the cell density was optimized beforehand to ensure HTRF detection within the dynamic range of the kit (data are shown for 200,000 cells/well)
The HTRF Total STAT5 assay efficiently detected endogenous STAT5 in various cellular models expressing different levels of the protein.
HTRF Total STAT5 assay compared to Western Blot
MOLT-4 cells were cultured in a T175 flask in complete medium at 37°C, 5% CO2. After medium removal, the cells were lysed with 3 mL of supplemented lysis buffer #4 (1X) for 30 min at RT under gentle shaking.
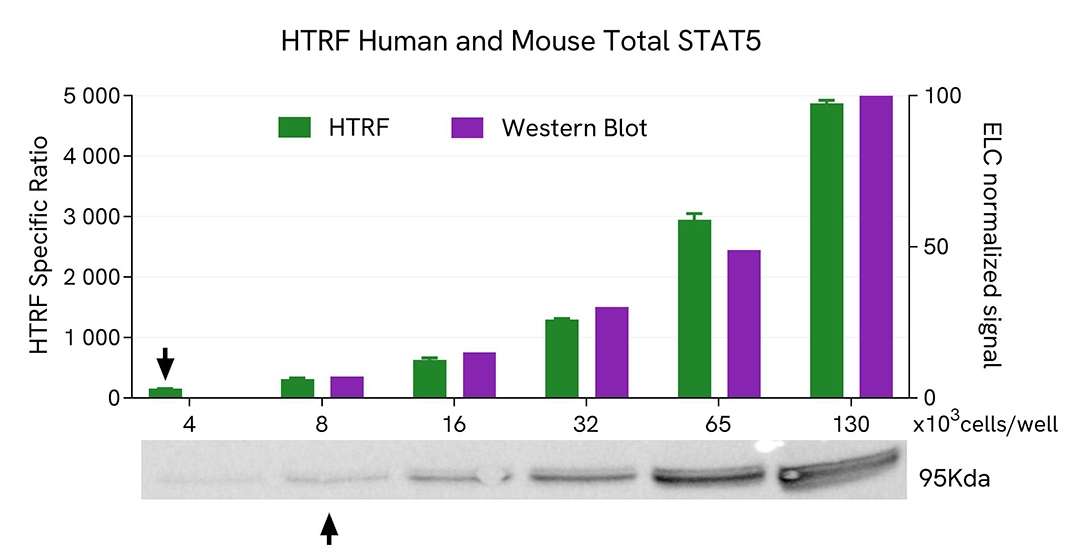
Serial dilutions of the cell lysate were performed using supplemented lysis buffer #4 (1X), and 16µL of each dilution were transferred into a 384-well small volume microplate before the addition of 4µL of HTRF Total STAT5 detection antibodies. HTRF signals were recorded after an overnight incubation.
Equal amounts of lysates were loaded into a gel for a side-by-side comparison between HTRF and Western Blot.
In these conditions, the HTRF Total STAT5 assay is 2-fold more sensitive than the Western Blot technique.
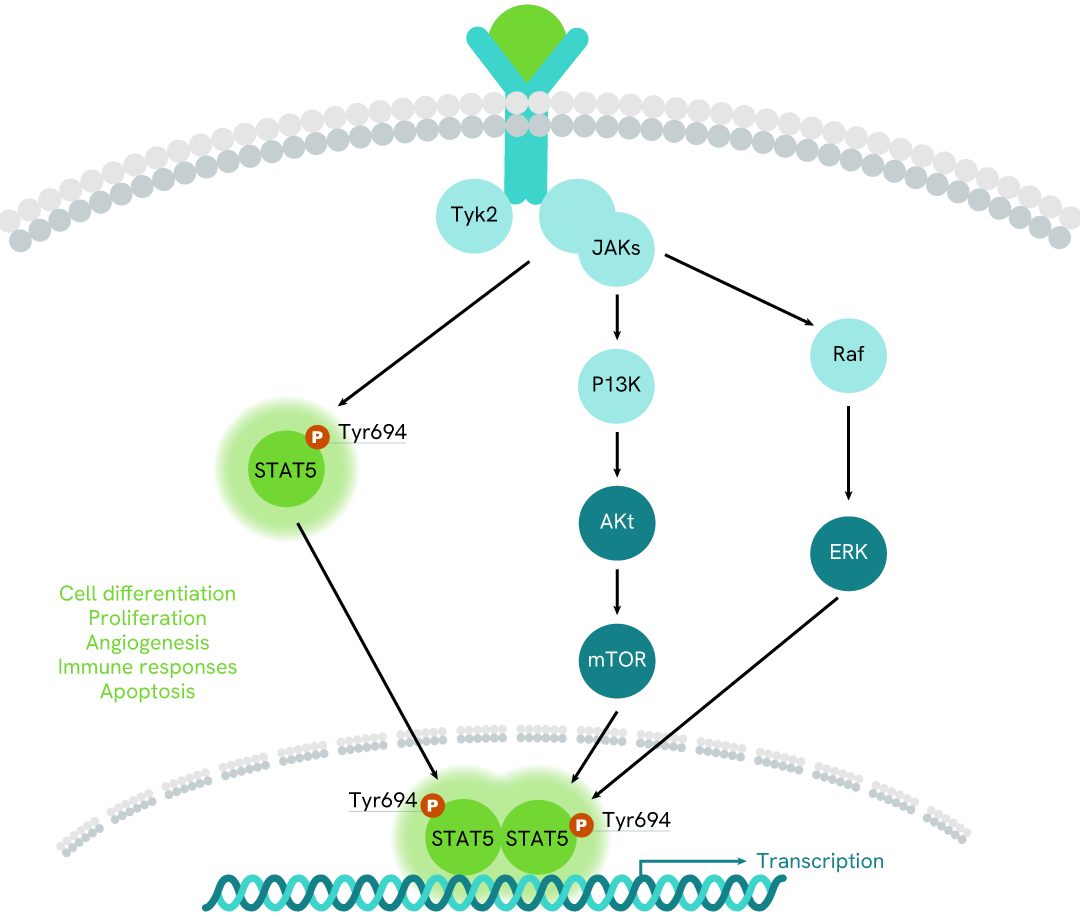
Simplified pathway
STAT5 signaling pathway
The activation of STAT5 proteins, is mediated by cytokines, and other growth factors, allowing rapid delivery of signals from the membrane to the nucleus. These STATs regulate the expression of target genes in a cytokine-specific fashion. In addition to their activation by cytokines, the activities of Stat5a and Stat5b are negatively controlled by CIS/SOCS/SSI proteins.
JAK/STAT pathway activation requires ligand binding to the receptor, which in consequence activates the Janus kinases JAKs, a family of intracellular tyrosine kinases. Via phosphorylation of downstream signal transducers and activators of transcription (STAT), STAT5 form homo or heterodimers (STAT5a and STAT5b) and translocate into the nucleus, where they bind to STAT5 response elements, inducing the transcription of specific sets of genes.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Buffer/Solvent |
Lysis Buffer 4
|
| Detection Modality |
HTRF
|
| Host Species |
Human
Mouse
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target |
STAT5
|
| Target Class |
Phosphoproteins
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Inflammation
Oncology
|
| Unit Size |
500 assay points
|
Resources
Are you looking for resources, click on the resource type to explore further.
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...


Loading...
How can we help you?
We are here to answer your questions.