

HTRF Human Total SMARCA-4 Detection Kit, 500 Assay Points







| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |









Product information
Overview
The SMARCA subgroup of genes belongs to the SWI/SNF family, involved in chromatin remodeling and DNA damage repair. SWI/SNF complexes regulate the genes involved in essential processes such as DNA replication, damage repair, and cell proliferation.
SMARCA4/BRG1 loss of function is frequently associated with cancer, and a synthetic lethal relationship between SMARCA4 and SMARCA2 /BRM is well established in SMARCA4-deficient cancers, where the inactivation of SMARCA2 leads to tumor cell death.
Among therapeutic strategies being investigated to fight cancers, targeted protein degradation is expected to overcome some limitations from conventional small molecules. So far, PROTAC molecules have been developed to induce selective degradation of SMARCA proteins. However, SMARCA2 and SMARCA4 share about 75% identity at the protein level, and so compounds are likely to target both proteins. Therefore, the selectivity of compounds targeting either SMARCA2 or SMARCA4 must be carefully investigated. In such investigations, the HTRF total SMARCA2 kit is a valuable companion assay for the HTRF total SMARCA4 kit, enabling reliable compound profiling.
How it works
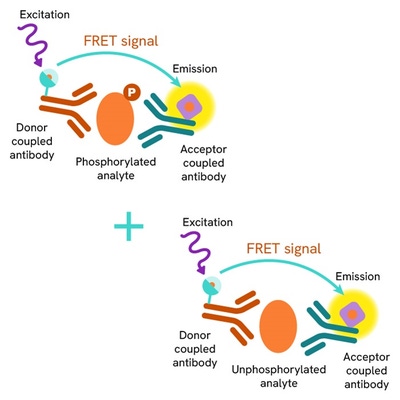
Total-SMARCA4 assay principle
The HTRF Total-SMARCA4 assay quantifies the expression level of SMARCA4 in a cell lysate. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The Total-SMARCA4 assay uses two labeled antibodies: one coupled to a donor fluorophore, the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In presence of SMARCA4 in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor, and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample, and provides a means of assessing the protein’s expression under a no-wash assay format.

Total-SMARCA4 two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of Total-SMARCA4 HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Total-SMARCA4 one-plate assay protocol
Detection of Total-SMARCA4 with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
Specificity of HTRF Total SMARCA4 assay using knockout HAP1 cell lines
SMARCA4 expression level was assessed with the HTRF total SMARCA4 kit in HAP1 cells (WT) and different HAP1 cell lines Knocked-Out for SMARCA4 or SMARCA2. Cell density was optimized beforehand to ensure HTRF detection within the dynamic range of the kit (data not shown).
The different cell lines were cultured in a 96-well plate (200,000 cells/well) for 24 hours at 37°C, 5% CO2. After culture medium removal, the cells were lysed with 50 µL of supplemented lysis buffer #1 (1X), then 16 µL of cell lysate were transferred into a low volume white microplate followed by 4 µL of premixed detection reagents. The HTRF signal was recorded after an overnight incubation at RT.
In HAP1 KO SMARCA4 cells, the HTRF signal was equivalent to the non-specific signal (dotted line), indicating a complete SMARCA4 gene silencing, whereas the SMARCA4 level was well detected in the other cell line, as expected.
Note that similar expression levels for SMARCA4 in WT and KO SMARCA2 cell lines demonstrate the selectivity of the HTRF SMARCA4 kit over SMARCA2, despite these two proteins sharing more than 70% sequence homology.
Catalog cell line references (Horizon Discovery): HAP1 Wt #C631; HAP1 KO SMARCA4 # HZGHC000922c003; HAP1 KO SMARCA2 # HZGHC004055c008

Validation on various Human and Mouse cell lines
The adherent Human cell lines HeLa (Cervix), HEK293 (Kidney), and the mouse cell line NIH3T3 were plated in 96-well culture plates at a density of 200,000 cells/well and incubated for 24 hours at 37°C, 5% CO2. After culture medium removal, the cells were lysed with 50 µL of supplemented lysis buffer #1 (1X) for 30 min at RT under gentle shaking.
The suspension Human cell lines HEL92.1.7 (erythroleukemia) and MOLT-4 (T lymphoblastic leukemia) were dispensed under 30 µL in a 96-well plate at a density of 200,000 cells/well, incubated for 1h at 37°C, 5% CO2, and lysed with 10 µL of supplemented lysis buffer #1 (4X) for 30 min at RT under gentle shaking.
The SMARCA4 expression levels were assessed with the HTRF Total SMARCA4 kit. Briefly, 16 µL of cell lysate were transferred into a low volume white microplate, followed by 4 µL of premixed HTRF detection reagents. The HTRF signal was recorded after an overnight incubation at RT. The dotted line corresponds to the non-specific HTRF signal. Note that the cell density was optimized beforehand to ensure HTRF detection within the dynamic range of the kit (data not shown).
the HTRF Total SMARCA4 assay efficiently detects endogenous SMARCA4 in various cellular models expressing different levels of the protein.

PROTAC® induced SMARCA4 degradation
HEL92.1.7 cells were seeded in a 96-well plate (100,000 cells/ 30µL well) and treated with increasing concentrations of the PROTAC® compounds ACBI1 and AU-15330, as well as a PROTAC® ARV-471 (PROTAC® Estrogen Receptor) used as a negative irrelevant control. After overnight incubation, cells were lysed with 10 µl of supplemented lysis buffer #1 (4X) for 30 min at RT under gentle shaking. After cell lysis, 16 µL of lysates were transferred into a 384-well low volume white microplate and 4 µL of the HTRF total SMARCA4 detection antibodies were added. The HTRF signal was recorded after an overnight incubation.
Cell treatment with the two SMARCA4 PROTAC® degraders triggered a dose-dependent decrease in SMARCA4 protein, while the expression levels of SMARCA4 in the ARV-471 control conditions as well as the ATP levels remained stable. These results suggest a PROTAC® induced specific SMARCA2 degradation, with a DC50* in the nM range, as expected (Farnabay, W. et al. Nat Chem Biol. 2019 and Xiao, L. et al. Nature 2022).
* DC50 corresponds to the concentration of the degrader at which 50% of the targeted protein is degraded.


PROTAC® SMARCA4 versus SMARCA2 profiling


The selective degradation of SMARCA2 in SMARCA4-mutated cancers induces synthetic lethality and is of major importance.
HEL92.1.7 cells (100,000 cells/well) were treated at 30 µL in a 96-well plate with increasing concentrations of the PROTAC® compounds ACBI1 and AU-15330. After overnight incubation, cells were lysed with 10 µl of supplemented lysis buffer #1 (4X) for 30 min at RT under gentle shaking. Following cell lysis, 16 µL of lysates were transferred into a 384-well low volume white microplate and 4 µL of the HTRF detection antibodies were added, using Total SMARCA2 or Total SMARCA4 assay reagents. The HTRF signal was recorded after an overnight incubation
These results indicate an ACBI1 and AU-15330 induced dose-dependent SMARCA2 and SMARCA4 decrease. ATP levels remained stable in the same conditions (data not shown). These results are consistent with studies from Farnabay, W. et al. (Nat Chem Biol. 2019) and Xiao, L. et al. (Nature 2022).
| PROTAC® | Described protein targets | E3L ligands | DC50 (nM) | |
| Total SMARCA2 | Total SMARCA4 | |||
| ACBI1 | SMARCA2 & SMARCA4 &PBRM1 | VHL Ligand |
224 |
231 |
| AU-15330 | SMARCA2 & SMARCA4 &PBRM1 | VHL Ligand |
242 |
620 |
HTRF total SMARCA4 assay compared to Western Blot
HEL92.1.7 cells were cultured in a T175 flask in complete culture medium at 37°C, 5% CO2. After 24h incubation and cell medium removal, the cells were lysed with 3 mL of supplemented lysis buffer #1 (1X) for 30 minutes at RT under gentle shaking.
Serial dilutions of the cell lysate were performed using supplemented lysis buffer#1, and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of HTRF total SMARCA4 detection reagents.
Equal amounts of lysates were used for a side-by-side comparison between HTRF and Western Blot.
In these conditions, the HTRF total SMARCA4 assay was 4-fold more sensitive than the Western Blot technique.

Simplified pathway
SMARCA4 Signaling Pathway
SMARCA2 and SMARCA4 are catalytic subunits within the SWI/SNF complex involved in DNA transcription and repair. SMARCA2 and SMARCA4 comprise 6 different domains, including a DNA-dependent ATPase domain and a Bromo domain. The Bromo domain and the H3K27 acetylated levels regulate the binding between DNA and the SWI/SNF complex, while the ATPase domain catalyzes the separation of the two DNA strands. These functions are counteracted by the EZH2 catalytic subunit of the PRC2 complex repressing gene transcription. The loss of function of SMARCA2 or SMARCA4 leads to increased DNA damage, as PRC2 continues to bind to the nucleosome and inhibit DNA repair.

Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
Lysis Buffer 2
Lysis Buffer 3
Lysis Buffer 4
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Unit Size |
500 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.


Loading...
How can we help you?
We are here to answer your questions.






























