

HTRF Human and Mouse Total Cofilin Detection Kit, 500 Assay Points







| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |









Product information
Overview
The kit is designed for the rapid detection of Total Cofilin in cell supernatant and whole cells. Actin-binding proteins are abundant cellular proteins that regulate cell function by mediating actin polymerization and remodeling. Cofilin, known as a regulator of actin filament dynamics, is a small ~21kDA protein that is ubiquitously expressed in all vertebrates and freely diffuses in eukaryotic cells.
How it works
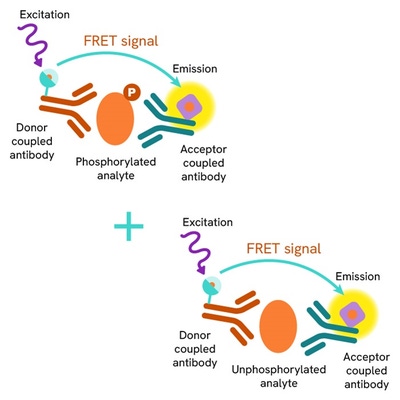
Total Cofilin assay principle
The HTRF Total Cofilin assay quantifyies the expression level of Cofilin. It is entirely plate-based and does not require gels, electrophoresis, or transfer. The assay uses two antibodies, one coupled to a donor fluorophore and the other to an acceptor. The two antibodies are highly specific for a distinct epitope on the protein. In presence of Cofilin in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor, and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample, and provides a means of assessing the protein’s expression under a no-wash assay format.

Total Cofilin two-plate assay protocol
The 2-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of the Total Cofilin HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Total Cofilin one-plate assay protocol
Detection of Total Cofilin with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS-designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
HTRF Phospho-Cofilin (Ser3) / Total Cofilin modulation using LIMK1 inhibitor and Staurosporin
HeLa cells were plated at 10,000 cell/well in complete culture medium, and incubated 24 hours at 37°C, 5% CO2. Cells were treated with increasing concentrations of Staurosporine or LIMK1 kinase (TH257) for respectively 1 or 2 hours at 37°C, 5% CO2. Supernatant was then discarded and 50µl of supplemented lysis buffer#4 were added to each well. The lysis step was done under gentle shaking for 30 minutes. Lysates were transferred into a 384 well plate under 16µl per well and 4µL of Total or Phospho Cofilin Ser3 detection reagents were dispensed. The plate was read after an overnight incubation.
As expected, both compounds induced a decrease in phosphorylated Cofilin at Ser3 upon treatment, while the Total Cofilin expression level was not modulated.


HTRF Phospho-Cofilin (Ser3) / Total Cofilin on neuronal cells
Human neuronal cells were plated at 10,000 cell/well in completed culture medium, and incubated 24 hours at 37°C, 5% CO2. Cells were treated with increasing concentrations of Staurosporine or LIMK1 kinase (TH257) for respectively 1 or 2 hours at 37°C, 5% CO2. Supernatant was then discarded, and 50µl of complemented lysis buffer#4 were added to each well. The lysis step was done under gentle shaking for 30 minutes. Lysates were transferred into a 384 well plate under 16µl per well and 4µL of Total or Phospho Cofilin Ser3 detection reagents were dispensed. The plate was read after an overnight incubation.
As expected, both compounds induced a decrease in phosphorylated Cofilin at Ser3 upon treatment, while the Total Cofilin expression level was not modulated.


HTRF Specificity of Total Cofilin assay using SiRNA
Hela cells were plated at 5,000 cell/well in complete culture medium, and incubated 24 hours at 37°C, 5% CO2. Cells were transfected with 50nmol of siRNA against Cofilin 1 and/or Cofilin 2 for 24 hours at 37°C, 5% CO2. Transfection medium was then removed, and cell culture medium was added on top of the cells for the next 24 hours at 37°C, 5% CO2. Supernatant was then discarded, and 50µl of supplemented lysis buffer#4 were added to each well. The lysis step was done under gentle shaking for 30 minutes. Lysates were transferred into a 384 well plate under 16µl per well and 4µL of total Cofilin Ser3 detection reagents were dispensed. The plate was read after an overnight incubation.
Cofilin 1 siRNA induced a 58% signal decrease in Total Cofilin 1 detection, while this was not the case for Cofilin 2. The data demonstrate that HTRF Total Cofilin 1 is specific for the detection of the Cofilin 1 protein and does not cross-react with Cofilin 2.

Total Cofilin assay versatility on human & mouse cell lines
Human HeLa and HepG2 cells and mouse NIH-3T3 cells were plated at 12,500 cell/well in complete culture medium, and incubated 24 hours at 37°C, 5% CO2. Supernatant was then discarded, and 50µl of supplemented lysis buffer#4 were added to each well. The lysis step was done under gentle shaking for 30 minutes. Lysates were transferred into a 384 well plate under 16µl per well and 4µL of HTRF total Cofilin detection reagents were dispensed. The plate was read after an overnight incubation.
Total Cofilin protein was efficiently detected in various human and mouse cellular models.

Comparison between HTRF and WB sensitivity in Total Cofilin assays
HeLa cells were cultured in a T175 flask in complete culture medium at 37°C, 5% CO2. After a 48h incubation, cells were lysed with 3mL of supplemented lysis buffer #4 (1X), for 30 minutes at RT under gentle shaking.
After a first dilution by 10, serial dilutions of the cell lysate were performed using supplemented lysis buffer, and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of HTRF Total Cofilin detection reagents. Equal amounts of lysates were used for a side-by-side comparison between HTRF and Western Blot.
In these conditions, the HTRF Total Cofilin assay is 16 times more sensitive than the Western Blot technique.

Simplified pathway
Cofilin Signaling Pathway
Actin-binding proteins are abundant cellular proteins that regulate cell function by mediating actin polymerization and remodeling. Cofilin, known as a regulator of actin filament dynamics, is a small ~21kDA protein that is ubiquitously expressed in all vertebrates and freely diffuses in eukaryotic cells. Cofilin promotes the conversion of actin filaments by enhancing F- actin depolymerization and inhibiting G-actin polymerization, which are essential in the actin filament dynamics of eukaryotes. Phosphorylation at Ser-3 by kinases attenuates cofilin’s actin- binding activity. In contrast, dephosphorylation at Ser-3 enhances cofilin-induced actin depolymerization. Cofilin functions are also modulated by various binding partners or reactive oxygen species.
Although the mechanism of cofilin-mediated actin dynamics has been known for decades, recent research studies have unveiled the profound impacts of cofilin dysregulation in neurodegenerative pathophysiology. For instance, an oxidative stress-induced increase in cofilin dephosphorylation has been linked to the accumulation of tau tangles and amyloid-beta plaques in Alzheimer’s Disease. In Parkinson’s Disease, cofilin activation by silencing its upstream kinases increases α-synuclein-fibril entry into the cell. Cofilin is also over expressed in cancer cells, promoting cell motility and acting as an important regulator of cancer metastasis.

Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
Lysis Buffer 2
Lysis Buffer 4
Lysis Buffer 5
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
TR-FRET
|
| Unit Size |
500 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.


Loading...
How can we help you?
We are here to answer your questions.






























