
HTRF Human and Mouse Total CK1a Detection Kit, 500 Assay Points
| Feature | Specification |
|---|---|
| Application | Protein Quantification |
| Sample Volume | 16 µL |



Product information
Overview
Casein kinase 1 (CK1) is a Serine/Threonine kinase that is ubiquitously expressed in eukaryotic organisms. Among its seven isoforms, CK1 alpha is involved in a wide range of cellular processes, including membrane trafficking, cell cycle progression, chromosome segregation, apoptosis, autophagy, cell metabolism, differentiation, circadian rhythm, the immune response, neurodegeneration, and cancer.
CELMoDs (Cereblon E3 Ligase Modulating Drugs), derived from IMIDs (immunomodulatory imide drugs), represent a promising therapeutic approach in cancer treatment, particularly in hematological malignancies, due to their mechanism as molecular glues. Notably, some CELMoDs, such as Iberdomide and CC-92480, specifically target CK1A by promoting its ubiquitination and degradation, thereby inhibiting cancer cell growth and proliferation.
How it works
Total CK1A assay principle
The Total CK1A assay quantifies the expression level of CK1A in a cell lysate. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The Total CK1A assay uses two labeled antibodies, one coupled to a donor fluorophore and the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In the presence of CK1A in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample and provides a means of assessing the protein's expression under a no-wash assay format.
Total CK1A two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of HTRF Total CK1A detection reagents. This protocol permits the cells' viability and confluence to be monitored.
Total CK1A one-plate assay protocol
Detection of Total CK1A with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol allows for miniaturization while maintaining robust HTRF quality.
Assay validation
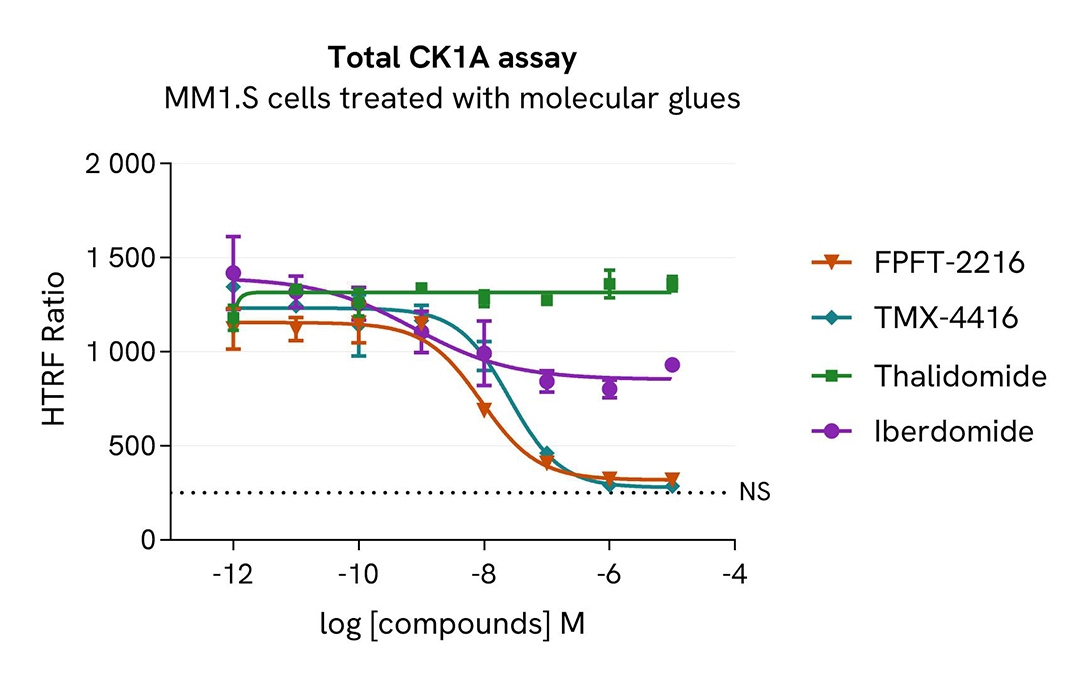
HTRF Total CK1A modulation using molecular glues on MM1.S cells
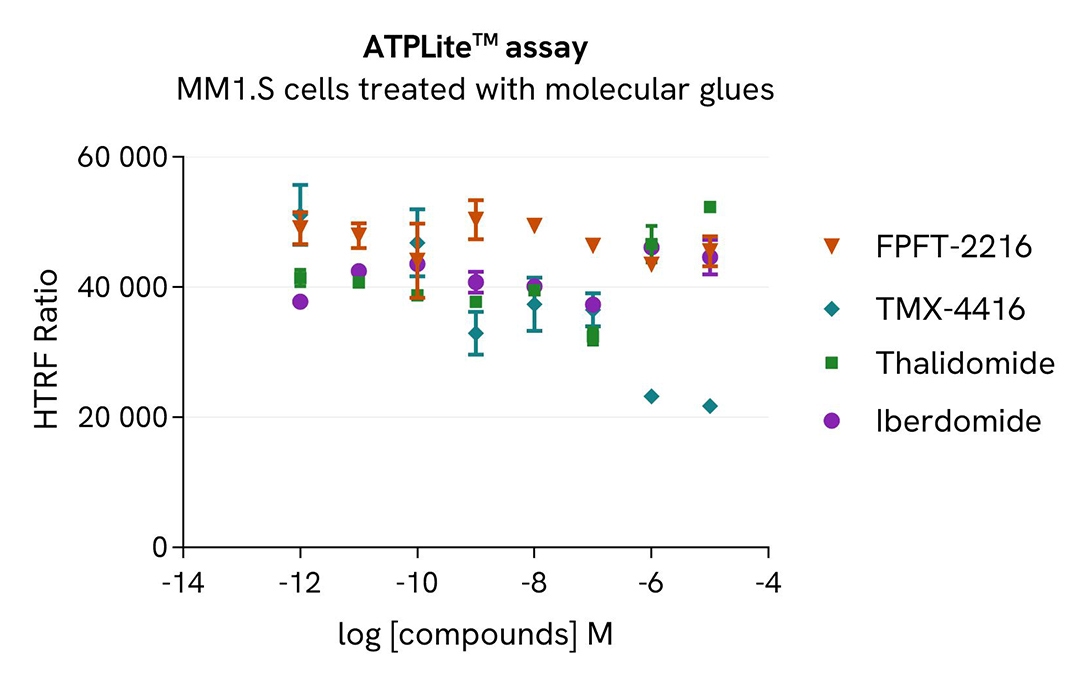
MM1.S cells were seeded in 96-well culture plate (100,000 cells/30µL well) and treated with increasing concentrations of the molecular glues FPFT-2216, TMX-4116, Thalidomide or Iberdomide.
After 24 hours incubation, cells were lysed with 10 µL of supplemented lysis buffer #4 (4X) for 30 min at RT under gentle shaking. Next, 16 µL of lysate were transferred into a 384-well low volume white microplate, and 4 µL of the HTRF Total CK1A detection antibodies were added. An additional volume of lysate were also transferred into the microplate to check the alpha-tubulin level and cytotoxicity with the alpha-tubulin housekeeping Cellular Kit (64ATUBPET/G/H) and ATPlite Luminescence Assay System ( #6016736) respectively . HTRF signals were recorded after an overnight incubation.
The three compounds FPFT-2216, TMX-4116 and Iberdomide triggered an CK1A dose-dependent decrease, whereas CK1A was not modulated with Thalidomide treatment in MM1.S cells. The alpha-tubulin expression level remained stable as expected. The ATP levels remained stable in cells treated with FPFT-2216, Iberdomide and Thalaidomide but decreased in cells treated with TMX-4116 indicating a possible cellular toxicity effect.
Our results indicate that FPFT-2216, TMX-4116 and Iberdomide induce CK1A degradation with higher potency (DC50* 9nM, 0,3nM and 77nM, respectively) and efficacy (95%, 97% and 42% degradation rate respectively).

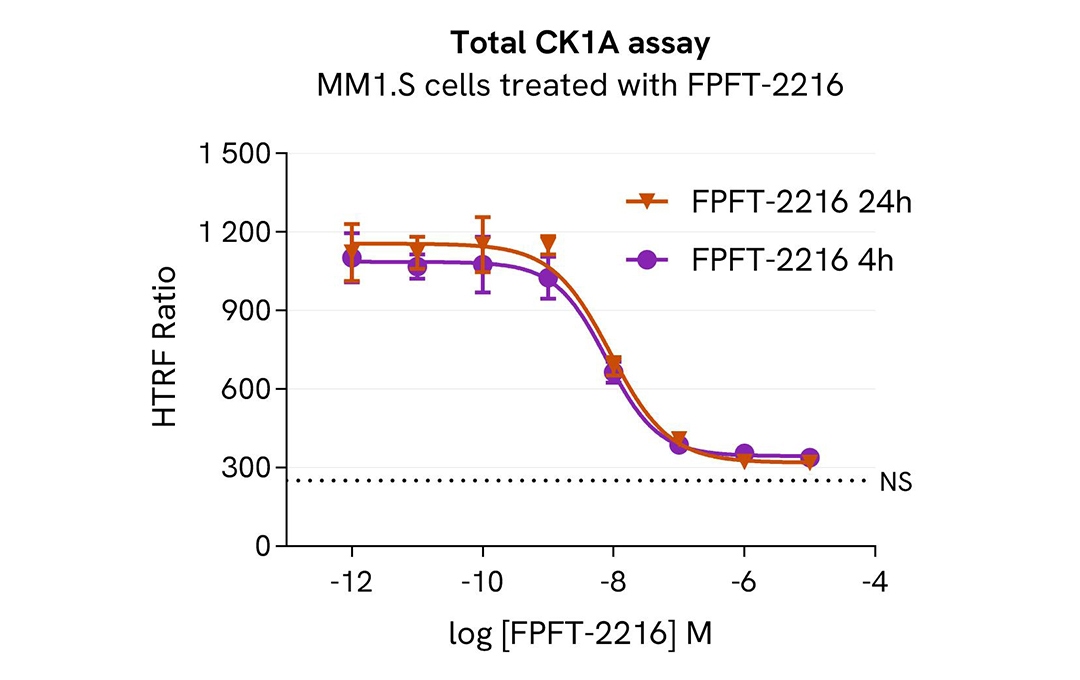
Total CK1A modulation kinetics using molecular glue
MM1.S cells were seeded in 96-well culture plate (100,000 cells/30µL well) and treated with increasing concentrations of the molecular glues FPFT-2216 for 4h or 24h.
After treatment, cells were lysed with 10 µL of supplemented lysis buffer #4 (4X) for 30 min at RT under gentle shaking. Next, 16 µL of lysate were transferred into a 384-well low volume white microplate, and 4 µL of the HTRF Total CK1A detection antibodies were added. An additional volume of lysate were also transferred into the microplate to check the alpha-tubulin level and cytotoxicity with the alpha-tubulin housekeeping Cellular Kit (64ATUBPET/G/H) and ATPlite Luminescence Assay System ( #6016736) respectively. HTRF signals were recorded after an overnight incubation.
The molecular glue FPFT-2216 induced a dose-dependent decrease in CK1A protein levels with DC50* of 8 nM and 9 nM after 4h and 24h, respectively.
* DC50 corresponds to the concentration of the degrader at which 50% of the targeted protein is degraded.
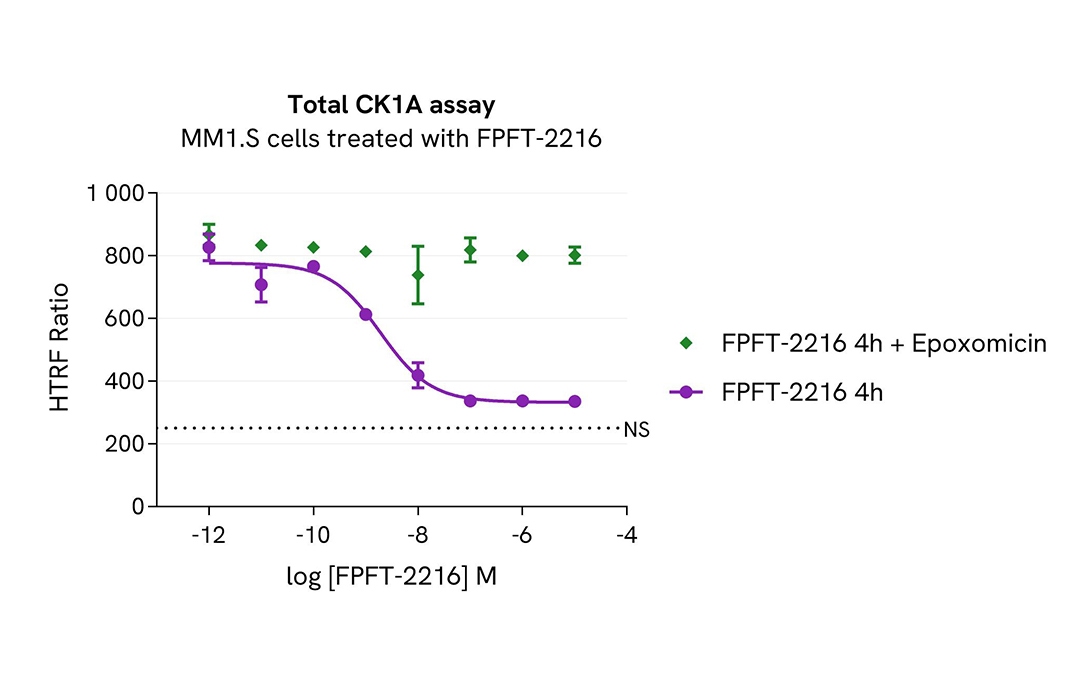
Total CK1A modulation using molecular glues is mediated by ubiquitin-proteasome system
MM1.S cells were seeded in 96-well culture plate (100,000 cells/30µL well) and treated with increasing concentrations of the molecular glues FPFT-2216 for 4h. The proteasome inhibitor epoxomicin (1µM) was added or not 1h prior to the addition of FPFT-2216.
After treatment, cells were lysed with 10 µL of supplemented lysis buffer #4 (4X) for 30 min at RT under gentle shaking. Next, 16 µL of lysate were transferred into a 384-well low volume white microplate, and 4 µL of the HTRF Total CK1A detection antibodies were added. An additional volume of lysate were also transferred into the microplate to check the alpha-tubulin level and cytotoxicity with the alpha-tubulin housekeeping Cellular Kit (64ATUBPET/G/H) and ATPlite Luminescence Assay System ( #6016736) respectively. HTRF signals were recorded after an overnight incubation.
FPFT-2216-induced CK1A degradation was prevented in the presence of epoxomicin, a proteasome activity inhibitor. This result unambiguously demonstrates that FPFT-2216-induced CK1A degradation is mediated by the ubiquitin proteasome system.
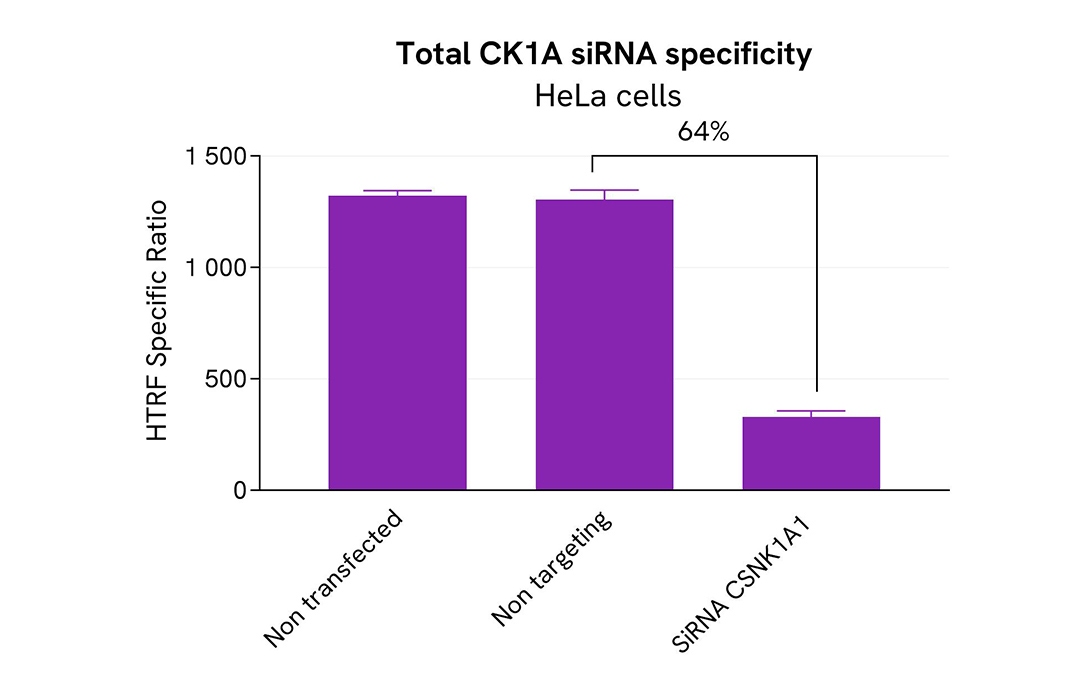
Validation of the specificity of Total CK1A assay using siRNA
HeLa cells were plated in a 96-well plate (50,000 cells/well) and cultured for 24h. The cells were then transfected with 50nM siRNA specific to CK1A as well as with a negative control siRNA. After 24 hours, the medium was replaced with fresh culture medium, and the cells were incubated for an additional 24 hours.
Post-treatment, the cells were lysed with 50 µL of supplemented lysis buffer #4 (4X) for 30 minutes at room temperature under gentle shaking. Subsequently, 16 µL of the lysates were transferred to a 384-well low-volume white microplate, and 4 µL of HTRF Total CK1A detection antibodies were added. HTRF signals were recorded after overnight incubation.
Transfection with CK1A specific siRNA resulted in 64% decrease in signal compared to cells transfected with the negative control siRNA. These results demonstrate the specificity of the HTRF total CK1A assay.
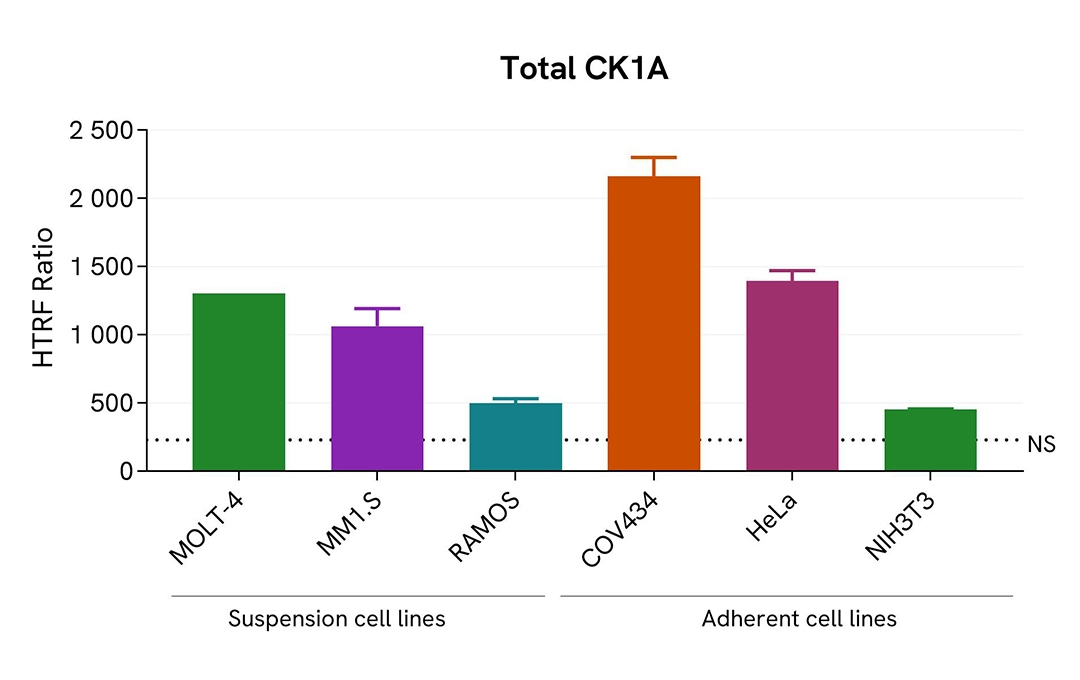
Assessment of Total CK1A level in various cell lines
The suspension cell lines (MOLT-4, Ramos & MM1.S) were dispensed into a 96-well plate and lysed with 10 µL of supplemented lysis buffer #4 (4X) for 30 min at RT under gentle shaking (performed according to the suspension cell protocol).
The adherent cell lines (HeLa, COV434 & NIH-3T3) were plated in 96-well culture plate and incubated for 24 hours at 37°C, 5% CO2. After medium removal, cells were lysed with 50 µL of supplemented lysis buffer #4 (1X) for 30 min at RT under gentle shaking.
The Total CK1A expression level was assessed with the HTRF Total CK1A kit. Briefly, 16 µL of cell lysate were transferred into a low volume white microplate, followed by 4 µL of premixed HTRF detection reagents. The HTRF signal was recorded after an overnight incubation at RT. The dotted line corresponds to the non-specific HTRF signal. Note that the cell density was optimized beforehand to ensure HTRF detection within the dynamic range of the kit (data are shown for 100,000 cells/well)
The HTRF Total CK1A assay efficiently detected endogenous CK1A in various cellular models expressing different levels of the protein.
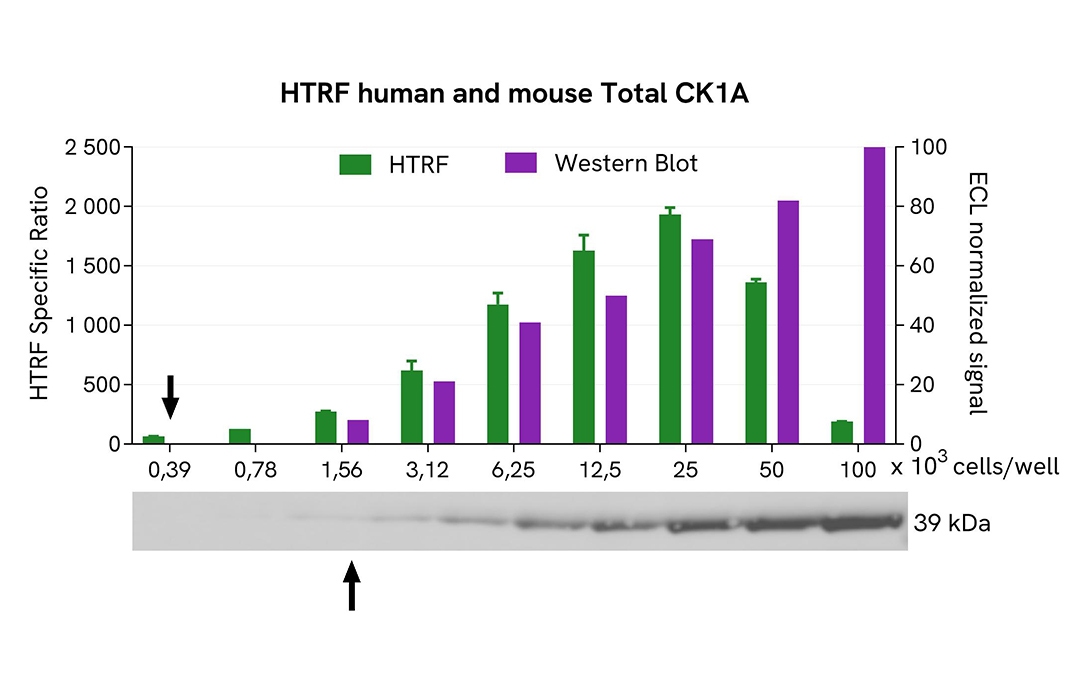
HTRF Total CK1A assay compared to Western Blot
HeLa cells were grown in a T175 flask in complete culture medium at 37°C - 5% CO2 until 80% confluence. After a 24h incubation, the cells were lysed with 3 mL of supplemented lysis buffer #4 (1X) for 30 minutes at RT under gentle shaking.
Serial dilutions of the cell lysate were performed using supplemented lysis buffer, and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of HTRF Total CK1A detection reagents. Equal amounts of lysates were used for a side-by-side comparison between HTRF and Western Blot.
In these conditions, the HTRF Total CK1A assay was 4 times more sensitive than the Western Blot technique.
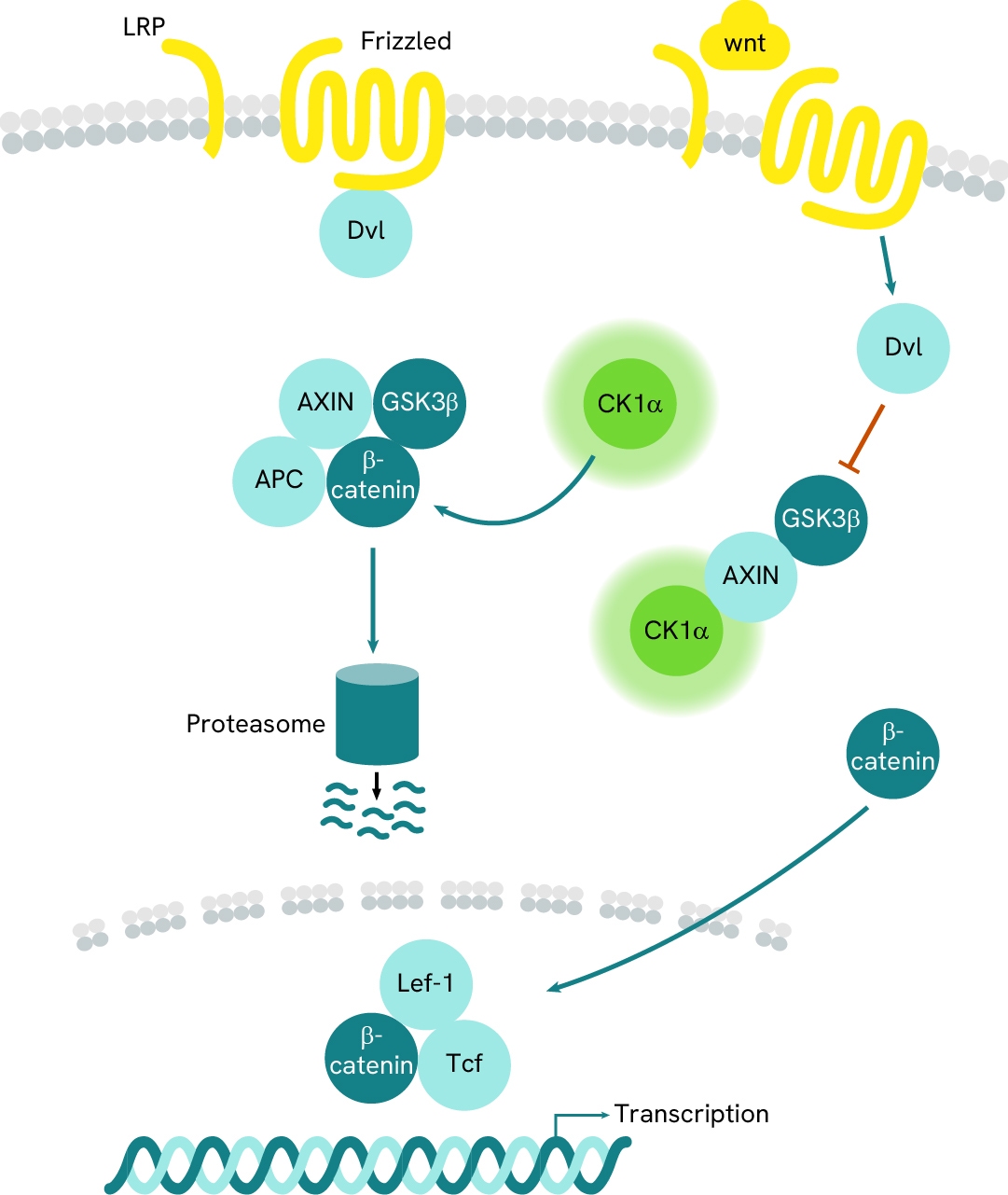
Simplified pathway
CK1A signaling pathway
In the absence of extracellular Wnt, CK1A phosphorylates β-catenin, which is part of a multi-protein complex, marking it for ubiquitination and subsequent degradation by the proteasome. Upon activation of the Frizzled receptor by extracellular Wnt ligands, Dishevelled (DVL) is phosphorylated and recruited to the receptor, leading to the inactivation of the GSK-3β/Axin/CK1A complex. This inactivation allows non-phosphorylated β-catenin to accumulate in the cytosol, where it then translocates to the nucleus to initiate gene transcription.
Specifications
| Application |
Protein Quantification
|
|---|---|
| Brand |
HTRF
|
| Buffer/Solvent |
Lysis Buffer 1
|
| Detection Modality |
HTRF
|
| Host Species |
Human
Mouse
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target |
CK1a
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Inflammation
Oncology
|
| Unit Size |
500 assay points
|
Resources
Are you looking for resources, click on the resource type to explore further.
This application note compares six CRBN modulators using no-wash HTRF assays to quantify degradation across key neosubstrates. The...


Loading...
How can we help you?
We are here to answer your questions.






























