HTRF Human Fibronectin EDA Detection Kit, 500 Assay Points





| Feature | Specification |
|---|---|
| Application | Protein Quantification |
| Sample Volume | 16 µL |









Product information
Overview
Fibronectin is a large glycoprotein of the extracellular matrix whose functions are connected to cell adhesion, growth, migration, and differentiation. Fibronectin level regulation is strongly related to inflammation and fibrotic disorders, as well as to the matrix re-organization that occurs in the environment of some cancers. Tumor containment, migration, and expansion are critically dependent on a tumor's surrounding matrix and on key adhesive and/or fibrous proteins such as fibroncetin.
The EDA domain of fibronectin is an alternatively spliced version of fibronectin that is almost entirely absent from healthy tissues, and mostly expressed in active fibroblasts at injury or inflammation sites. Because of this special feature, EDA-fibronectin is a marker that is specific to fibroblast-induced fibrotic disorders, as well as being a readout of pro-fibrotic activity in a tumor matrix environment.
How it works
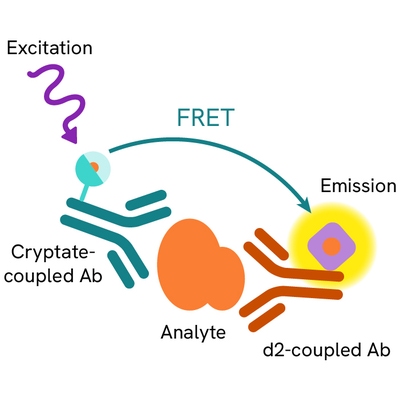
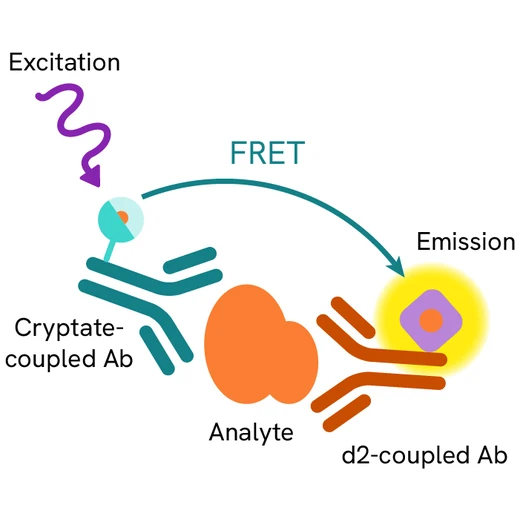
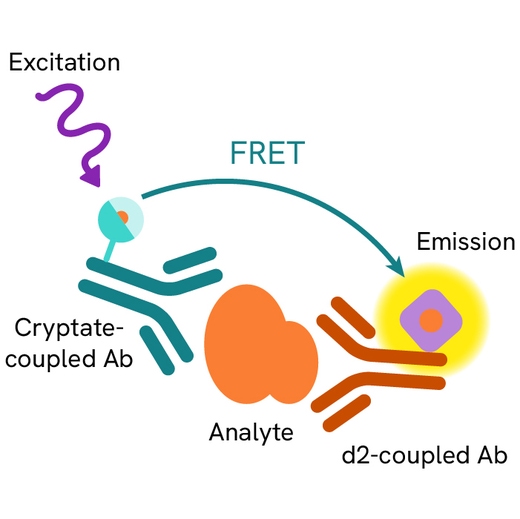
Human Fibronectin EDA assay principle
Human Fibronectin EDA is measured using a sandwich immunoassay involving specific anti-Fibronectin EDA antibodies, respectively labelled with Europium Cryptate (donor) and d2 (acceptor). The intensity of the signal is proportional to the concentration of Fibronectin EDA present in the sample.

Human Fibronectin EDA assay protocol
The Human Fibronectin EDA assay protocol is shown here using a 384-well small volume white plate (20 µL final). Cell supernatant, whole cell lysate, sample, or standard is dispensed directly into the assay plate for the detection of Fibronectin EDA by HTRF reagents. The antibodies labelled with HTRF fluorophores may be pre-mixed and added in a single dispensing step to further streamline the assay procedure. The assay can be run in 96- to 384-well plates by simply resizing each addition volume proportionally.

Assay details
Human Fibronectin EDA assay specifications
| Sample size | 16 µL |
|---|---|
| Final assay volume | 20 µL |
| Time to results |
ON at RT (or after 4h, possible) |
| Detection limit | 5.2 ng/mL |
| Dynamic range | 12 - 1,500 ng/mL |
| Sample compatibility | Cell supernatant and Cell Lysate |

Analytical performance
Precision
| Sample | Mean [Fibronectin EDA] (ng/mL) | CV |
|---|---|---|
| 1 | 50 | 4% |
| 2 | 180 | 3% |
| 3 | 750 | 3% |
Each of the 3 samples was measured 24 times, and the %CV was calculated for each sample.
| Sample | Mean [Fibronectin EDA] (ng/mL) | CV |
|---|---|---|
| 1 | 226 | 7% |
| 2 | 649 | 8% |
Each of the samples was measured in 4 independent experiments by 4 different operators, and the % CV was calculated for each sample.
Antigen spike and recovery
| Sample | [Standard Fibronectin EDA] (ng/mL) | [Native Fibronectin EDA] added (ng/mL) | Proportion (%) Standard/Native | [Fibronectin EDA] expected (ng/mL) | [Fibronectin EDA] measured (ng/mL) | Antigen Recovery |
|---|---|---|---|---|---|---|
| 1 | 181 | 0 | 100/0 | 90.5 | 92 | 101% |
| 2 | 181 | 221 | 45/55 | 201 | 212 | 105% |
| 3 | 181 | 548 | 25/75 | 364.5 | 357 | 98% |
|
4
|
181 | 926 | 15/85 | 553.5 | 594 | 107% |
| 5 | 522 | 0 | 100/0 | 261 | 253 | 97% |
| 6 | 522 | 221 | 70/30 | 371.5 | 375 | 101% |
| 7 | 522 | 548 | 50/50 | 535 | 499 | 93% |
| 8 | 522 | 926 | 35/65 | 724 | 737 | 102% |
Two levels of recombinant protein (~180 and 520 ng/mL) were added to 3 dilutions of native sample from primary human lung fibroblast cell lysates and the expected concentrations were compared to those measured in order to compute antigen recoveries (acceptance criteria: 90-110%). The recovery was good after overnight incubation at RT.
Two levels of recombinant protein (~180 and 520 ng/mL) were added to 3 dilutions of native sample from primary human lung fibroblast supernatants, and the expected concentrations were compared to those measured in order to compute antigen recoveries (acceptance criteria: 90-110%). The recovery was good after overnight incubation at RT.
Dilutional linearity
| Dilution Factor | C obtained (ng/ml) | C expected (ng/ml) | % Recovery |
|---|---|---|---|
| Neat | 769.8 | 769.8 | 100% |
| 2 | 361.72 | 384.88 | 94% |
| 4 | 176.54 | 192.44 | 92% |
| 8 | 81.60 | 96.22 | 85% |
| 16 | 46.44 | 48.11 | 97% |
| 32 | 19.86 | 24.06 | 83% |
Samples were from primary human lung fibroblast cell extracts diluted in the kit diluent Lysis Buffer 3 (1X). The recovery % obtained from these experiments showed the good dilutional linearity of the assay (dilution test acceptance criteria: 80-120%).
| Dilution Factor | C obtained (ng/ml) | C expected (ng/ml) | % Recovery |
|---|---|---|---|
| Neat | 888.38 | 888.38 | 100% |
| 2 | 423.79 | 444.19 | 95% |
| 4 | 212.88 | 222.10 | 96% |
| 8 | 106.89 | 111.05 | 96% |
| 16 | 57.50 | 55.52 | 104% |
| 32 | 31.93 | 27.76 | 115% |
Samples were from primary human lung fibroblast supernatant diluted in the kit diluent Lysis Buffer 3 (1X). The recovery % obtained from these experiments showed the good dilutional linearity of the assay (dilution test acceptance criteria: 80-120%).
Cross reactivities & Interference
| Recombinant Peptide | Reactivity |
|---|---|
| Plasmatic Fibronectin EDA- | 0.23% at 5000 ng/ml |
| FCS (10%) | No |
Cross reactivities were determined by using standard curves for recombinant peptides in the kit diluent Lysis Buffer 3 (1X), with concentrations ranging from 0 to 1500 ng/mL or up to 10% of Fetal calf serum (FCS). No significant cross-reactivity was observed.
| Recombinant Peptide | Interference (5000 ng/ml) |
|---|---|
| Integrin α1β5 | No |
| Collagen | No |
| Anastellin | No |
| Heparin | No |
Interference was tested by spiking 6 increasing concentrations of peptides (up to 5000 ng/mL) in a sample containing a Fibronectin EDA concentration of 1000 ng/mL. No significant interference was observed.
Assay validation
Human Fibronectin EDA expression upon differentiation of fibroblast into myofibroblasts
Primary human lung fibroblasts (HLFs) were plated in a culture-treated 96-well plate (12,500 cells/well) in complete culture medium and incubated at 37°C - 5% CO2. The day after, the cells were treated with increasing concentrations of TGF-ß1 for 48 hours in serum-free culture medium supplemented with 1% BSA. After supernatant collection, the cells were lysed with 50 µL of lysis buffer #3 for 30 minutes at RT under gentle shaking.
16 µL of lysate or supernatant diluted in lysis buffer #3 to remain in the linear range of the assay were transferred into a low volume white microplate before the addition of 4 µL of the HTRF Human Fibronectin EDA detection antibodies. The HTRF signal was recorded after an overnight incubation. TGF-ß1 treatment results in a dose dependent increase of Fibronectin EDA expression level, which demonstrates the differentiation of fibroblasts into myofibroblasts.


Human Fibronectin EDA expression in cancer cell line after TGFβ1 treatment
The adenocarcinomic human alveolar basal epithelial cells, A549, was plated in a culture-treated 96-well plate (50,000 cells/well) in complete culture medium and incubated at 37°C - 5% CO2. The day after, the cells were treated with 10 ng/ml of TGF-ß1 for 48 hours in serum-free culture medium supplemented with 1% BSA. The supernatant was collected, and cells were lysed with 50 µL of lysis buffer #3. 16 µL of lysate or supernatant were transferred into a low volume white microplate before the addition of 4 µL of the HTRF Human Fibronectin EDA detection antibodies. The HTRF signal was recorded after an overnight incubation.
TGF-ß1 treatment results in an increase of Fibronectin EDA expression level in lysate and supernatant, which demonstrates an upregulation of an epithelial to mesenchymal transition (EMT) marker, as expected.

Simplified pathway
EDA fibronectin roles and regulation
Fibronectin (FN) is the main adhesive protein bridging the gap between cells and the extracellular matrix (ECM). It is typically secreted by fibroblasts as part of their ECM elaboration role, and is critical to morphogenesis, tissue architecture, and wound repair.
The EDA domain of fibronectin is an alternatively spliced domain that is largely absent from normal adult tissues. Its presence is characteristic of specific tissue remodeling events including injury, inflammation, wound repair, scarring, and fibrosis. Because of this, EDA fibronectin is a key and early marker of fibroblast activation when stress, growth factors, cytokines, and tissue stiffness promote their differentiation into active myofibroblasts. Along with a-SMA contractile fibers and increased collagen excretion, EDA fibronectin is a hallmark of active fibroblasts.
Unlike regular fibronectin, the EDA domain is known to amplify inflammation and fibrotic behavior in at least two ways. The EDA domain interacts with latent TGF-b binding protein-1 (LTBP-1) which in turn stores TGF-β1 in the immediate proximity of the fibroblasts drawn to the site and increases their differentiation into contractile collagen-secreting myofibroblasts. EDA fibronectin also promotes inflammatory pathways in fibroblasts, mastocytes, T-cells, and monocytes by triggering toll-like receptors TLR4.
The current understanding is that EDA-Fn splicing is regulated by the P13K/AKT pathway as a result of TGF-b and other modulators, or via regular FN signaling or mechanical tension through integrins.

Specifications
| Application |
Protein Quantification
|
|---|---|
| Automation Compatible |
Yes
|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
Lysis Buffer 2
Lysis Buffer 3
Lysis Buffer 4
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Biomarkers
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Inflammation
NASH/Fibrosis
Oncology & Inflammation
|
| Unit Size |
500 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.
Guide to How Cellular Actors of the Tumor Microenvironment Challenge Immunotherapies
Since the early 2000s, significant scientific...


Loading...
How can we help you?
We are here to answer your questions.