

HTRF Human Total EGFR L858R Detection Kit, 500 Assay Points

 View All
View All








| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |















Product information
Overview
Drug resistance in patients with EGFR mutated non-small cell lung cancer treated with tyrosine kinase inhibitors (TKIs) is one of the main challenges for cancer targeted therapy. Designing specific PROTAC compounds to degrade EGFR mutated proteins is expected to offer a promising approach to treating such patients, and overcome drug resistance.
How it works
Total-EGFR L858R assay principle
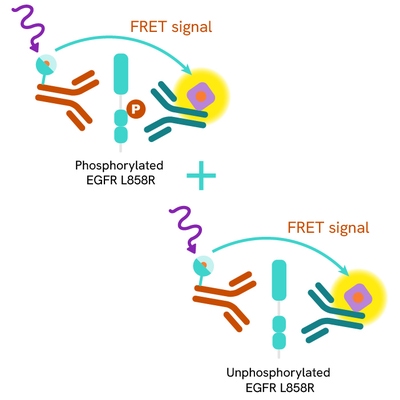
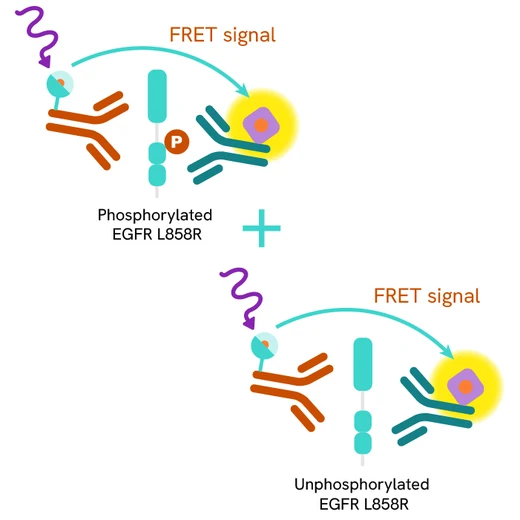
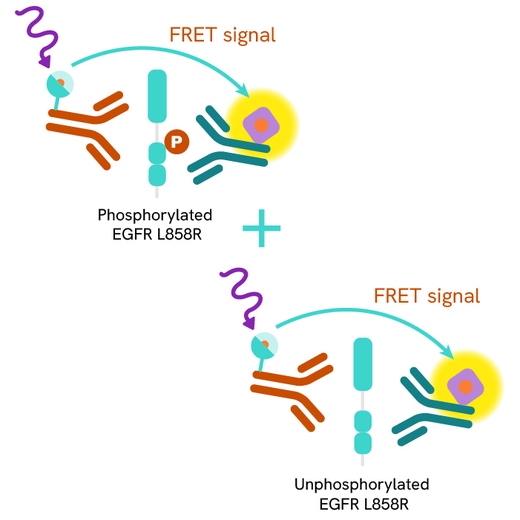
The HTRF Total-EGFR L858R assay quantifies the expression level of mutated EGFR L858R in a cell lysate. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The Total-EGFR Mutant L858R assay uses two labeled antibodies: one coupled to a donor fluorophore, the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In presence of EGFR Mutant L858R in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor, and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample, and provides a means of assessing the protein’s expression under a no-wash assay format.

Total-EGFR L858R two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of Total-EGFR L858R HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored

Total-EGFR L858R one-plate assay protocol
Detection of Total-EGFR Mutant L858R with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
PROTAC® compounds induce the degradation of mutant EGFR L858R
H3255 cells were seeded in a 96-well culture-treated plate under 20,000 cells / well in complete culture medium, and incubated overnight at 37° C, 5% CO2.The cells were then treated with increasing concentrations of PROTAC® (MS39-SJF1528-Gefitinib based PROTAC 3-MS154 and SJF1521) for 16H at 37°C, 5% CO2. After cell lysis with 50 µL of supplemented lysis buffer #4, 8 µL of lysates were transferred into a 384-well low volume white microplate before the addition of 8µL of supplemented lysis buffer #4 and 4 µL of HTRF Total EGFR L858R detection antibodies. The HTRF signal was assessed after an overnight incubation.
As expected, the results obtained show a dose-dependent decrease in mutant EGFR L858R in the H3255 cells induced by the PROTAC® molecules, and the DC50* were assessed for each compound. In the same experimental conditions, no cytotoxic effects were observed, as evidenced by constant ATP levels measured with ATPliteTM assay.
DC50 corresponds to the concentration of the degrader at which 50% of the targeted protein is degraded.


Specificity of the Total EGFR L858R kit assessed by siRNA
H1975 cells were plated in 96-well plates (20,000 cells/well) and cultured for 24h. The cells were then transfected with siRNAs specific for EGFR, ERBB2, ERBB3, and ERBB4, as well as with a negative control siRNA. After a 48h incubation, the cells were lysed and 16 µL of lysates were transferred into a 384-well low volume white microplate before the addition of 4 µL of the HTRF Total EGFR L858R detection antibodies.
EGFR siRNA led to a 79% signal decrease compared to the cells transfected with the negative siRNA. No signal decrease was observed in cells transfected with ERBB2, ERBB3, or ERBB4 siRNAs. In the same experimental conditions, the silencing of the EGFR members had no effect on cell proliferation or viability, as evidenced by constant ATP levels measured with the ATPliteTM assay.
These results demonstrate that the HTRF Total EGFR L858R is specific and does not detect other HER/ErbB family members.


Total EGFR L858R assay validated in relevant EGFR mutated cell lines.
Total EGFR L858R expression levels were assessed with the HTRF Total EGFR DEL19 kit in different cell lines: H1975 and H3255 cells (expressing EGFR L858R mutant), A431 and A549 cells (expressing wild type EGFR), and H1650 and HCC827 (expressing the EGFR DEL19 mutant).
The different cell lines were cultured in T175 flasks in complete culture medium at 37°C, 5% CO2. After a 48h incubation, the cells were lysed with 3 mL of supplemented lysis buffer #4 (1X) for 30 minutes at RT under gentle shaking. 16 µL of each lysate diluted by 2 were transferred into a low volume white microplate before the addition of 4 µL of HTRF total EGFR L858R detection reagents.
As expected, no signal was detected in the H1650, HCC827, A431, and A549 cell lines, demonstrating the specificity of the HTRF Total EGFR L858R kit to detect mutated EGFR L858R only.

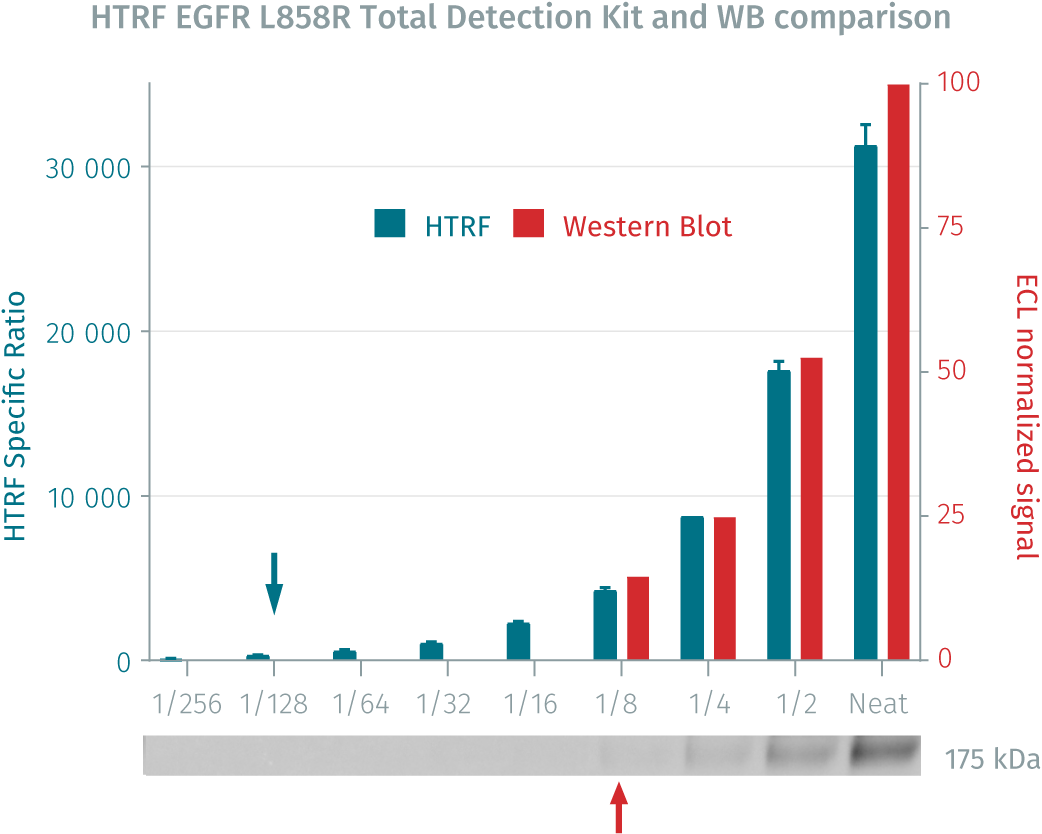
HTRF Total EGFR L858R assay compared to Western Blot
H1975 cells were cultured in a T175 flask in complete culture medium at 37°C, 5% CO2. After a 48h incubation, the cells were lysed with 3 mL of supplemented lysis buffer #4 (1X) for 30 minutes at RT under gentle shaking.
Serial dilutions of the cell lysate were performed using supplemented lysis buffer, and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of HTRF total EGFR L858R detection reagents. Equal amounts of lysates were used for a side-by-side comparison between HTRF and Western Blot.
In these conditions, the HTRF total EGFR L858R assay is 17 times more sensitive than the Western Blot technique.
Simplified pathway
Total EGFR Signaling Pathway
EGFR is a receptor tyrosine kinase that belongs to the ErbB family. Binding of EGFR ligands drives receptor homodimerization or hetero-dimerization, leading to the activation of the EGFR tyrosine kinase domain and specific tyrosine residues.
The phosphorylated tyrosine residues then provide docking sites for a variety of factors that induce downstream activation of several signal transduction cascades.
The signals transmitted from the EGF receptor to the nucleus lead to the regulation of various biological functions, such as cell proliferation, differentiation, survival, adhesion, migration, and angiogenesis.
Mutations that lead to overexpression or hyperactivity of EGFR are associated with a number of cancers, making EGFR a key target for anti-cancer therapies.

Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
Lysis Buffer 2
Lysis Buffer 3
Lysis Buffer 4
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Unit Size |
500 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
Read this brochure to get more information about the chemagic Prime Jr intrument for automated nucleic acid purification and assay...
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.


Loading...
How can we help you?
We are here to answer your questions.






























