

HTRF Human and Mouse Total FAK Detection Kit, 500 Assay Points







| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |









Product information
Overview
The Total FAK cellular assay monitors total FAK and is used as a normalization assay with the phospho-FAK kit. This kit is compatible with the buffers from the phospho-FAK kit, so the same lysate can be used for analyses of both the phosphorylated and the total protein populations.
FAK, Focal Adhesion Kinase, is a cytoplasmic tyrosine kinase controlling cell adhesion, migration, proliferation, and survival. FAK is a central regulator of ECM/integrin and cadherin signaling, and plays a role in cell adhesion, cell junction, migration, survival, and mechanosensing. FAK can be activated by Growth Factor Receptors, Cytokine Receptors, and GPCRs. Increased FAK expression and phosphorylation is associated with increased tumor cell adhesion, migration, invasion in several cancers, and with fibroblast migration on fibronectin in tissue fibrosis.
How it works
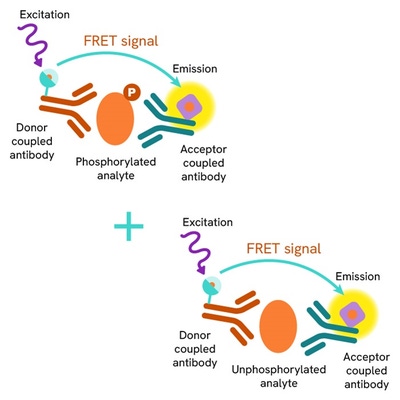
Total-FAK assay principle
The Total FAK assay quantifies the expression level of FAK in a cell lysate. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The Total-FAK assay uses two labeled antibodies, one coupled to a donor fluorophore, the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In presence of FAK in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample, and provides a means of assessing the protein's expression under a no-wash assay format.

Total-FAK two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates to a 384-well low volume detection plate before the addition of Total-FAK HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Total-FAK one-plate assay protocol
Detection of Total FAK with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
Accumulation of phosphorylated FAK (Tyr397) in human cancer cell lines
Human MCF7 or HeLa cells were plated in a 96-well plate (100,000 cells/well) in complete culture medium and incubated at 37°C, 5% CO2. The day after, cells were treated for 10 min in the presence or absence of Pervanadate. After medium removal, cells were lysed with 50 µL of supplemented lysis buffer #3 for 30 minutes at RT under gentle shaking, and 16 µL of lysate were transferred twice over into a low volume white microplate before the addition of 4 µL of the premixed HTRF phospho-FAK (Tyr397) or Total-FAK detection reagents. The HTRF signal was recorded after an overnight incubation.
Treatment with pervanadate, a phosphatase inhibitor, lead to a significant increase in the phosphorylation of FAK on Tyrosine 397. The total amount of FAK protein remained unaffected by the pervanadate treatment.


HTRF Phospho and Total FAK assays to monitor pharmacology of FAK inhibitors
NIH/3T3 mouse fibroblasts or human HeLa cells were plated in a 96-well plate (100,000 cells/well) in complete culture medium and incubated at 37°C, 5% CO2. The day after, cells were treated with increasing concentrations of FAK inhibitors for 30 min. After medium removal, cells were then lysed with 50 µL of supplemented lysis buffer #3 for 30 minutes at RT under gentle shaking, and 16 µL of lysate were transferred twice over into a low volume white microplate before the addition of 4 µL of the premixed HTRF phospho-FAK (Tyr397) or Total-FAK detection reagents. The HTRF signal was recorded after an overnight incubation.
Treatment with PF-562271 lead to a dose dependent decrease in FAK phosphorylation on Tyrosine 397, in both Human HeLa and Mouse NIH/3T3 cell lines. The total amount of FAK protein remained unaffected by the pervanadate treatment.


HTRF FAK assays as readouts to monitor growth factor receptor activation
HUVEC human endothelial cells were plated in a 24-well collagen I plate (400,000 cells/well) in complete culture medium and incubated at 37°C, 5% CO2. The day after, cells were incubated in serum-free conditions. The following day, cells were treated with increasing concentrations of VEGF for 5 min in serum-free conditions. After medium removal, cells were then lysed with 250 µL supplemented lysis buffer #3 for 30 minutes at RT under gentle shaking, and 16 µL of lysate were transferred twice over into a low volume white microplate before the addition of 4 µL of the premixed HTRF phospho-FAK (Tyr397) or Total-FAK detection reagents. The HTRF signal was recorded after an overnight incubation.
HUVEC cells stimulated with VEGF showed a dose dependent increase in FAK phosphorylation on Tyrosine 397. VEGF regulates FAK phosphorylation and plays a role in angiogenesis and cell growth.

HTRF phospho-FAK cellular assays compared to Western Blot
The mouse NIH/3T3 cell line was seeded in a T175 flask in complete culture medium, and incubated for 2 days at 37°C, 5% CO2, until 90% confluency was reached. Cells were then lysed with 3 mL of supplemented lysis buffer #3 for 30 minutes at RT under gentle shaking. Soluble supernatants were collected after a 10-minute centrifugation.
Serial dilutions of the cell lysate were performed in the supplemented lysis buffer, and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of HTRF® phospho-FAK detection reagents. Equal amounts of lysates were used to establish a side-by-side comparison between HTRF and Western Blot.
Using the HTRF® Total-FAK kit, just 2,500 cells/well were sufficient to detect a significant signal while 5,000 cells were needed for minimal ECL signal detection using Western Blot. The HTRF assay is therefore twice as sensitive as the Western Blot technique.

Simplified pathway
FAK simplified pathway
This multi-domain protein changes conformation upon activation by cell surface receptors, mainly by integrins, cytokine receptors (IL1βR, TNFαR), growth factor receptors (EGFR, VEGFR, PDGFR), and GPCRs (LPA, bombesin). The cytoplasmic inactive FAK monomer is recruited to the plasma membrane by activated receptors, that yield to a transient dimerization and subsequent activation by autophosphorylation on tyrosine 397. This is a docking site for Src family kinases, allowing phosphorylation of other residues. Fully activated FAK displays binding sites for FAK substrates including Grb2, PI3K, and cadherins. FAK serves as a central regulator of ECM/integrin signaling, is required in the regulation of assembly/disassembly of focal adhesions, and plays a role in cell adhesion, migration, survival, and mechanosensing. In cadherin signaling, the regulation of adherens junctions between endothelial/epithelial cells enables FAK to play a role in cell junction, vascular permeability, and angiogenesis. FAK can activate various substrates and regulate multiple signaling pathways, such as Rho GTPases/actin (cytoskeleton reorganization/cell migration), Cadherin/β-catenin (cell junctional stability/vascular permeability), PI3K/AKT (cell survival), MAPKs ERK & JNK (cell proliferation), and p53/MDM2 (anti-apoptosis).

Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
Lysis Buffer 2
Lysis Buffer 3
Lysis Buffer 4
Lysis Buffer 5
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Cardiovascular
NASH/Fibrosis
Oncology & Inflammation
|
| Unit Size |
500 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.


Loading...
How can we help you?
We are here to answer your questions.






























