

HTRF Human and Mouse Total CDK2 Detection Kit, 500 Assay Points







| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |









Product information
Overview
The Total CDK2 cellular assay monitors total CDK2, and can be used as a normalization assay with our phospho-CDK2 (Tyr15) kit. This kit is compatible with the buffers from the phospho-CDK2 kit, so the same lysate can be used for analyses of both the phosphorylated and the total protein populations.
CDK2 (Cyclin-Dependent Kinase 2) is a member of the subfamily of CDKs that coordinate cell cycle progression in mammalian cells (including also CDK1, CDK4, and CDK6). CDK2 is activated by interaction with Cyclin E and Cyclin A during the late G1 phase and the S phase respectively, and is also regulated by two major phospho-sites. Phosphorylation at Tyr15 by the kinase Wee1 is inhibitory by preventing ATP binding, whereas Tyr15 dephosphorylation by the phosphatase Cdc25A and phosphorylation at Thr160 by CAK (CDK Activating Kinase) is required for its full activity.
CDK2 inhibitory phosphorylation at Tyr15 is essential for maintaining genome integrity and preventing DNA damage during the S phase. The Wee1/Cdc25A axis is therefore an attractive target for cancer therapy and may represent a unique approach to sensitize cancer cells with hyperactive CDK2.
How it works
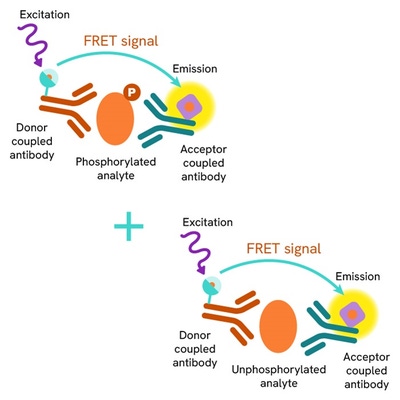
Total CDK2 assay principle
The Total CDK2 assay quantifies the expression level of CDK2 in a cell lysate. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The Total CDK2 assay uses two labeled antibodies, one coupled to a donor fluorophore and the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In the presence of CDK2 in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample, and provides a means of assessing the protein's expression under a no-wash assay format.

Total CDK2 two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of Total CDK2 HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.
Total CDK2 one-plate assay protocol
Detection of Total CDK2 with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
Validation of the specificity of Total CDK2, Total CDK4, and Total CDK6 assays by siRNA experiments
HeLa and HEK293 cells were plated in 96-well plates (40,000 and 50,000 cells/well respectively) and cultured for 24h. The cells were then transfected with siRNAs specific for CDK1, CDK2, CDK4, or CDK6, as well as with a negative control siRNA. After 48h incubation, the cells were lyzed and 16 µL of lysates were transferred into a 384-well low volume white microplate before the addition of 4 µL of the HTRF Total CDK2, Total CDK4, or Total CDK6 detection antibodies. The HTRF signal was recorded after an overnight incubation.
Cell transfection with each specific CDK2, CDK4, or CDK6 siRNA led to 77 to 97% signal decrease compared to the cells transfected with the negative siRNA. It should be noted that the small signal decrease observed on total CDK6 assay when cells were transfected with CDK2 siRNA was expected, since CDK2 knockdown leads to down-regulation of CDK6 (Bačević et al., Sci Rep. 2017; 7: 13429). Taken together, these data demonstrate that HTRF Total CDK2, Total CDK4, and Total CDK6 assays are specific for each kinase and do not cross-react with other cell cycle CDK family members.



Dephosphorylation of CDK2 Tyr15 by alkaline phosphatase
HeLa cells were cultured in a T175 flask for 48h and treated with 0.5 µg/mL Aphidicolin for 20h. The cells were then lyzed with 3 mL of supplemented lysis buffer #2 (1X) and only a part of the lysate was treated with alkaline phosphatase (AP). For the detection step, 16 µL of lysates were transferred into a 384-well low volume white microplate and 4 µL of the HTRF Phospho-CDK2 (Tyr15) or Total CDK2 detection antibodies were added. The HTRF signal was recorded after an overnight incubation.
As expected, alkaline phosphatase induced the nearly complete dephosphorylation of CDK2 on Tyr15, while the total level of the kinase was not modulated by the treatment.

Phospho-CDK2 (Tyr15) modulation using cell cycle blockers
Human HeLa cells and mouse NIH-3T3 cells were cultured in a 96-well plate (50,000 cells/well) for 24h and then treated for 16h with the cell cycle blockers Aphidicolin and Nocodazole respectively.
After cell lysis, 16 µL of lysates were transferred into a 384-well low volume white microplate and 4 µL of the HTRF Phospho-CDK2 (Tyr15) or Total CDK2 detection antibodies were added. The HTRF signal was recorded after an overnight incubation.
As expected, Aphidicolin (which arrests cells in the early S phase) induced a dose-dependent accumulation of phosphorylated CDK2 at the inhibitory site Tyr15.
Inversely, Nocodazole (which arrests cells at the G2/M border) induced a dose-dependent decrease in CDK2 phosphorylation on Tyr15.
The expression level of the kinase remained relatively stable, whatever the cell treatment.


Phospho-CDK2 (Tyr15) inhibition using Wee1 kinase inhibitors
HeLa cells were cultured in a 96-well plate (50,000 cells/well) for 24h and then treated for 2h with the Wee1 kinase inhibitors Adavosertib and PD0166285.
After cell lysis, 16 µL of lysates were transferred into a 384-well low volume white microplate and 4 µL of the HTRF Phospho-CDK2 (Tyr15) or Total CDK2 detection antibodies were added. The HTRF signal was recorded after an overnight incubation.
As expected, both Wee1 kinase inhibitors triggered a dose-dependent decrease in phosphorylated CDK2 at Tyr15, while the expression level of the protein was not modulated by the treatments.


HTRF total CDK2 assay compared to Western Blot
HeLa cells were cultured in a T175 flask in complete culture medium at 37°C-5% CO2. After 48h incubation, the cells were lyzed with 3 mL of supplemented lysis buffer #2 (1X) for 30 minutes at RT under gentle shaking.
Serial dilutions of the cell lysate were performed using supplemented lysis buffer, and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of HTRF total CDK2 detection reagents. Equal amounts of lysates were used for a side by side comparison between HTRF and Western Blot.
Using the HTRF total CDK2 assay, 800 cells/well were enough to detect a significant signal, while 1,600 cells were needed to obtain a minimal chemiluminescent signal using Western Blot. Therefore in these conditions, the HTRF total CDK2 assay was twice as sensitive as the Western Blot technique.

Simplified pathway
Role of CDK2 in the cell-division cycle
CDK2 (Cyclin-Dependent Kinase 2) is a member of the subfamily of CDKs that coordinate cell cycle progression in mammalian cells (also including CDK1, CDK4, and CDK6).
Mitogenic signals, such as growth factors, trigger cells to enter the G1 phase of the cell cycle by inducing cyclin D synthesis, leading to the formation of active CDK4/6-cyclin D complexes. CDK4 and CDK6 mono-phosphorylate the protein of retinoblastoma (RB), which still binds to transcription factor E2F, but some genes can be transcribed, such as cyclin E. In the late G1 and early S phases, Cyclin E interacts with and activates CDK2, which in turn phosphorylates additional sites on RB resulting in its complete inactivation. The E2F-responsive genes required for S phase progression are thus induced, such as Cyclin A which then interacts with CDK2 to form Cyclin A/CDK2 complexes. Activated CDK2 finally phosphorylates Cdc25B & Cdc25C phosphatases, which in turn activate CDK1, required for progression in the G2 and M phases of the cell-division cycle.

Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 2
Lysis Buffer 3
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Oncology & Inflammation
|
| Unit Size |
500 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.
An in-depth review of molecular and cellular pathways
The maintenance of proteostasis, the biological mechanisms that control the...


Loading...
How can we help you?
We are here to answer your questions.






























