Supporting lentiviral vector development for cell therapies and beyond
Lentiviral vectors are an efficient tool for fast, efficient, and cost-effective generation of stable homogeneous cell models for strong gene expression or gene knockdown for diverse research and discovery projects. In gene therapy, lentiviruses are widely used for CAR-T cell therapy applications.
Lentiviral vector manufacturing
Revvity offers a variety of lentiviral vector manufacturing options (USP and DSP) that are optimized for minimal process and product related impurities for research studies, discovery programs or preclinical projects. During the project set-up process our experts work with you to tailor the process to your project needs, e.g.
- screening of multiple target sequences
- generation of test model systems
- understanding of a biological mode of action
- characterizing lead vector manufacturability in a preclinical pilot run
For research use only. Not for use in diagnostic procedures.
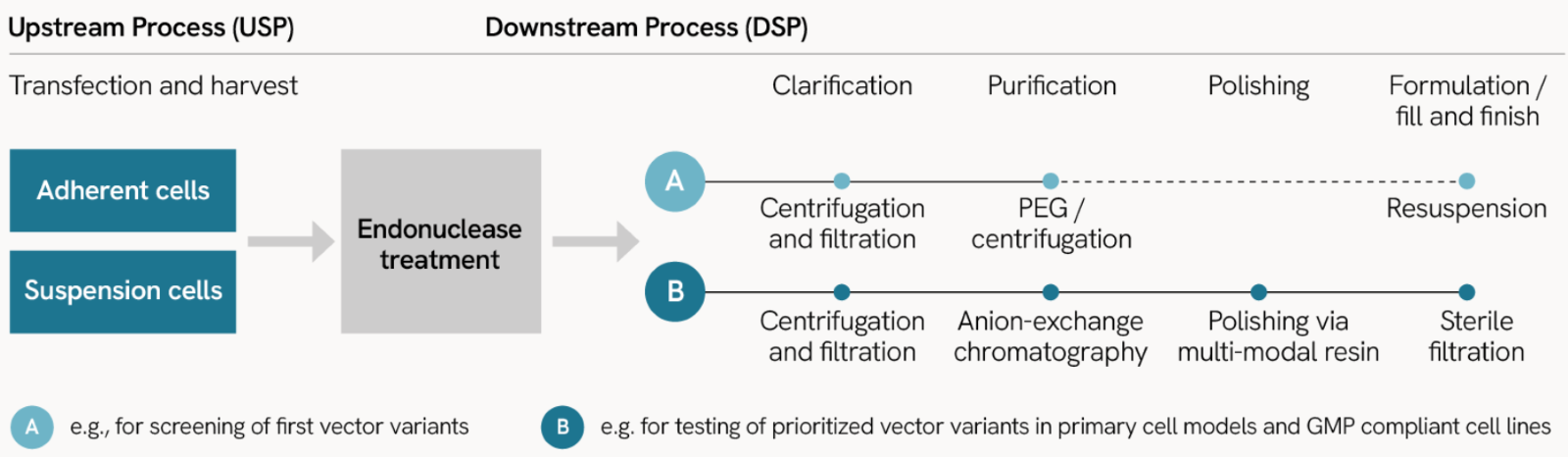
Manufacturing of lentiviral particles for research and discovery follows our ISO certified manufacturing procedure
Upstream processes
- Utilizing adherent or suspension cells for lentiviral vector production with a 3rd generation LV vector system based on 3’SIN Technology, and pseudotyped with VSV-G protein.
Downstream processes
Flexible purification options:
- Standard purification: PEG precipitation and ultracentrifugation, ideal for screening vector variants or discovery projects.
- High quality purification: Affinity chromatography purification and ultracentrifugation for higher purity, ideal for preclinical applications.
Quality control overview
- Our Quality Control (QC) packages include various assays to assess the potency of LV vectors, such as genome titration (qPCR) and functional titration (transduction units and infectious units). Additional assays and purity checks, such as sterility and mycoplasma tests or residual host cell proteins and DNA, are available upon request.
Our experienced process development team also establishes advanced lentiviral vector manufacturing procedures on demand for individual or preclinical requirements for experimental validation and the experimental workflow.
Revvity’s gene delivery key advantage
De-risking: All our lentivirus production projects profit from 20 years of in-house expertise in lentivirus vector technology.
Integrated development: We carefully consider the deep connection between the lead vector design and any challenge encountered during the development of a cell and gene therapy, including quality, potency and cost effectiveness.
Needs and technology focus: Our specialists are experienced in supporting cell therapy developers by carefully discussing and identifying our clients’ needs. Project feasibility as well as a portfolio of development and manufacturing options are addressed in a comprehensive project set-up process.
Lentiviral vectors for cell and gene therapy
Drawing from many years of experience providing lentiviral vector production services, Revvity Gene Delivery (formerly SIRION Biotech) provides a 360° end-to-end solution within the R&D and preclinical lentivirus space to develop safe and efficacious lentivirus-based drug products.
We offer capabilities and technologies to develop the key components of a lentivirus-based drug product:
- Therapeutic expression cassette development
- Lentiviral surface modification
- Manufacturing process tools and development
- Patented transduction enhancer technology LentiBOOST
Lentiviral vector manufacturing process
Manufacturing of lentiviral particles for research follows our ISO certified manufacturing procedure.
Our experienced process development team also establishes advanced manufacturing procedures on demand for individual requirements for experimental validation and the experimental workflow.
GMP alliances
Our preclinical lentiviral vector manufacturing processes – including custom developed advanced manufacturing procedures – are transferrable to partners within our closely aligned, worldwide CDMO network for GMP-manufacturing. This significantly speeds up the transfer process. We can additionally transfer the plasmid sequences for LV drug substance manufacturing and provide physical plasmid starting material via plasmid CDMO partners.