
AlphaLISA SureFire Ultra Human and Mouse Phospho-STAT1 (Tyr701 ) Detection Kit, 500 Assay Points
 View All
View All










| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 10 µL |





















Product information
Overview
STAT1 (Signal Transducer and Activator of Transcription 1) is a crucial transcription factor in the JAK/STAT signaling pathway, playing a central role in both human and mouse cellular processes. STAT1 is particularly important in mediating interferon responses, immune regulation, and cell growth control. Activation of STAT1 occurs through phosphorylation at a key tyrosine residue, Tyr701. This phosphorylation is essential for STAT1 dimerization, nuclear translocation, and subsequent transcriptional activity. STAT1 signaling is frequently implicated in various physiological and pathological conditions, including viral infections, inflammatory diseases, and certain types of cancer. Aberrant activation or suppression of the STAT1 pathway can contribute to disease progression, immune dysregulation, and therapy resistance.
The AlphaLISA SureFire Ultra Human and Mouse Phospho-STAT1 (Tyr701) Detection Kit is a highly sensitive sandwich immunoassay designed for the quantitative detection of phosphorylated STAT1 (Tyr701) in cellular lysates from both human and mouse samples, using Alpha technology.
Formats:
- The HV (high volume) kit contains reagents to run 100 wells in 96-well format, using a 60 μL reaction volume.
- The 500-point kit contains enough reagents to run 500 wells in 384-well format, using a 20 μL reaction volume.
- The 10,000-point kit contains enough reagents to run 10,000 wells in 384-well format, using a 20 μL reaction volume.
- The 50,000-point kit contains enough reagents to run 50,000 wells in 384-well format, using a 20 μL reaction volume.
AlphaLISA SureFire Ultra kits are compatible with:
- Cell and tissue lysates
- Antibody modulators
- Biotherapeutic antibodies
Alpha SureFire kits can be used for:
- Cellular kinase assays
- Receptor activation studies
- Screening
How it works
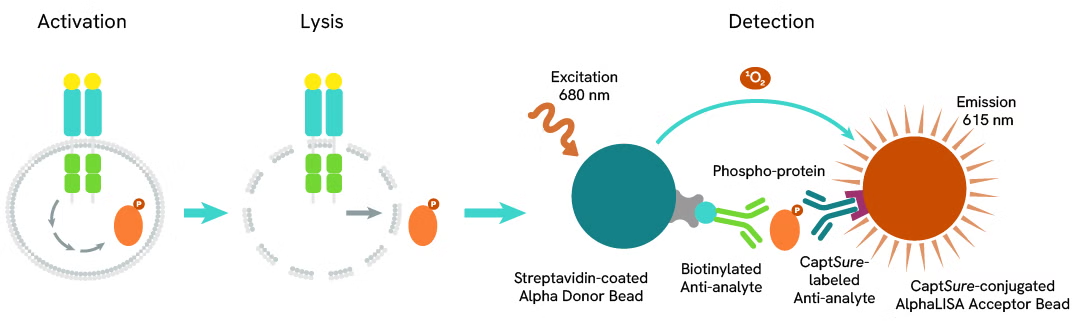
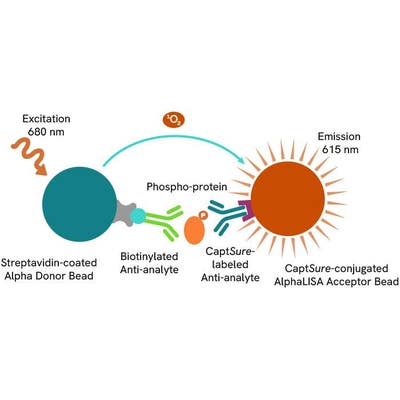
Phospho-AlphaLISA SureFire Ultra assay principle
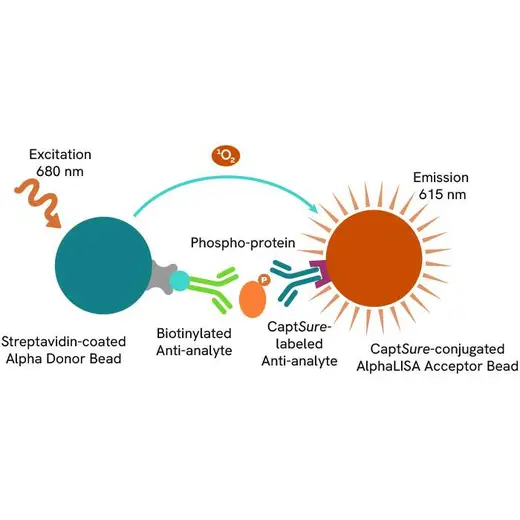
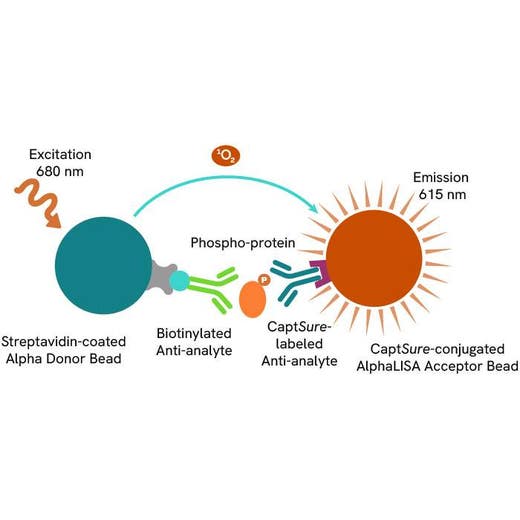
The Phospho-AlphaLISA SureFire Ultra assay measures a protein target when phosphorylated at a specific residue.
The assay uses two antibodies which recognize the phospho epitope and a distal epitope on the targeted protein. AlphaLISA assays require two bead types: Acceptor and Donor beads. Acceptor beads are coated with a proprietary CaptSure™ agent to specifically immobilize the assay specific antibody, labeled with a CaptSure tag. Donor beads are coated with streptavidin to capture one of the detection antibodies, which is biotinylated. In the presence of phosphorylated protein, the two antibodies bring the Donor and Acceptor beads in close proximity whereby the singlet oxygen transfers energy to excite the Acceptor bead, allowing the generation of a luminescent Alpha signal. The amount of light emission is directly proportional to the quantity of phosphoprotein present in the sample.

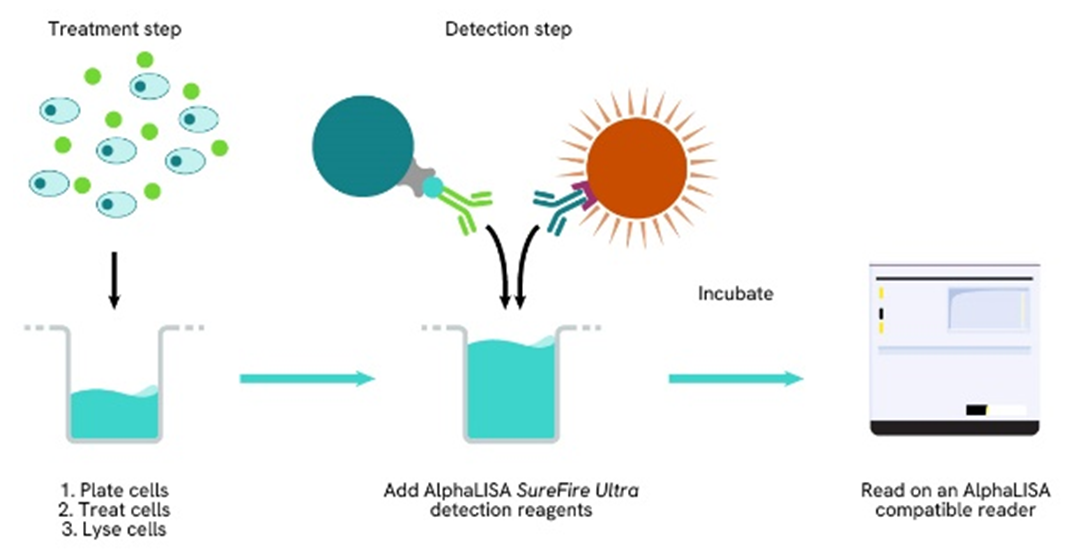
Phospho-AlphaLISA SureFire Ultra two-plate assay protocol
The two-plate protocol involves culturing and treating the cells in a 96-well plate before lysis, then transferring lysates into a 384-well OptiPlate™ plate before the addition of Phospho-AlphaLISA SureFire Ultra detection reagents. This protocol permits the cells viability and confluence to be monitored. In addition, lysates from a single well can be used to measure multiple targets.
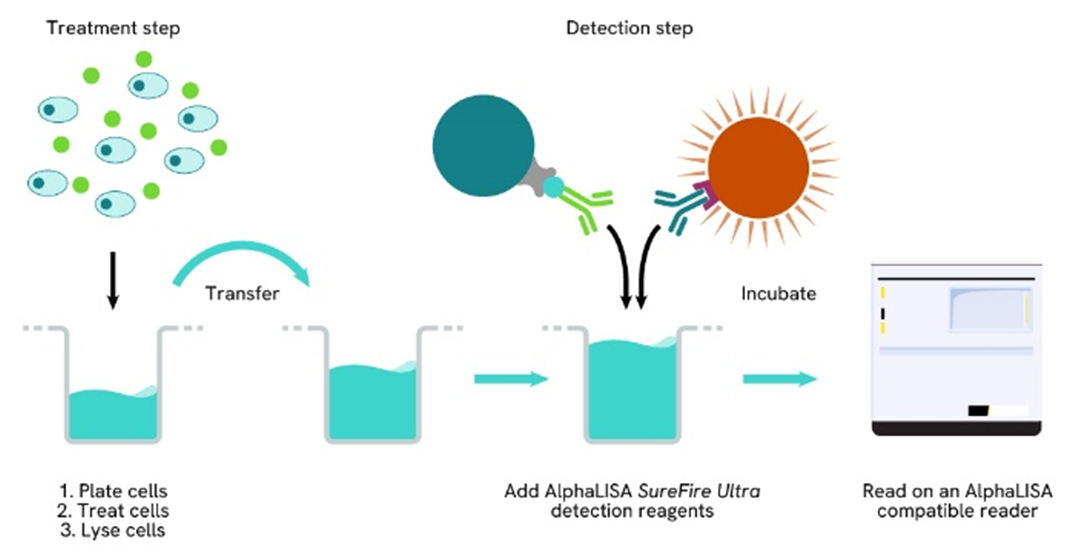
Phospho-AlphaLISA SureFire Ultra one-plate assay protocol
Detection of Phosphorylated target protein with AlphaLISA SureFire Ultra reagents can be performed in a single plate used for culturing, treatment, and lysis. No washing steps are required. This HTS designed protocol allows for miniaturization while maintaining AlphaLISA SureFire Ultra quality.
Assay validation
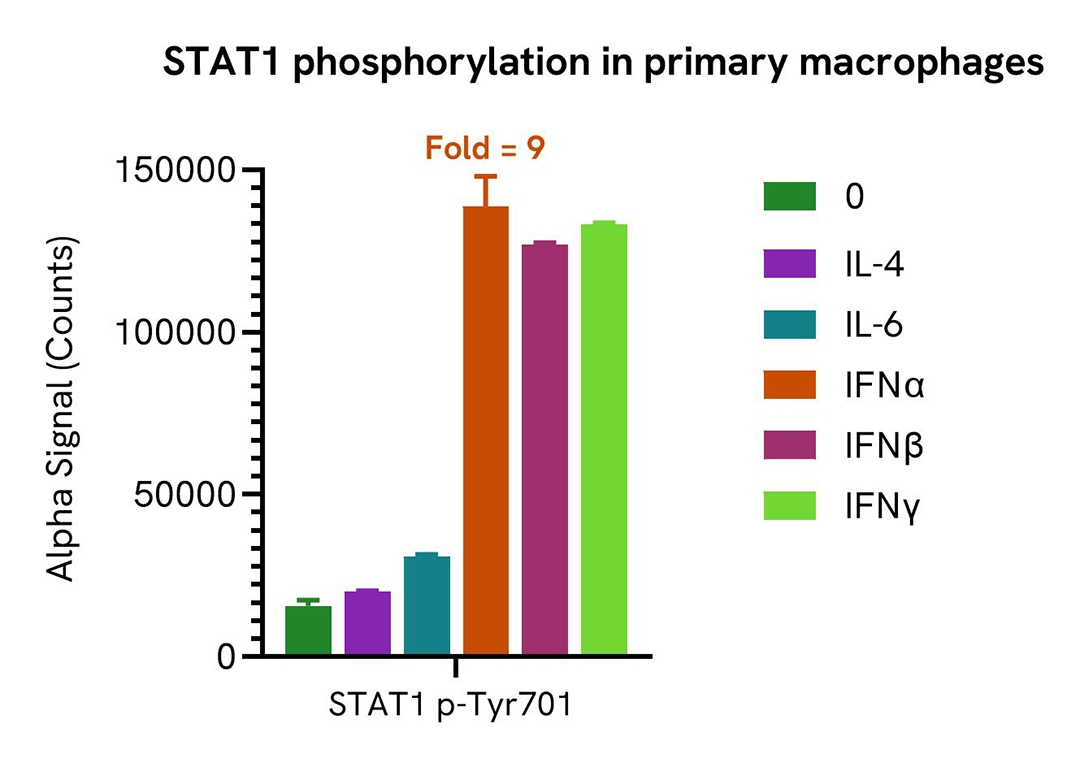
Induction of STAT1 (Tyr701) phosphorylation in primary macrophages treated with various cytokines
PBMCs were isolated from healthy donors and cultured for 6 days in complete DMEM containing 20 ng/mL M-CSF to differentiate them into macrophages. Macrophages were seeded in a 96-well plate (40,000 cells/well) in complete DMEM, and incubated overnight at 37°C, 5% CO2. Cells were starved for 2 h and then treated with the indicated cytokines for 15 minutes.
After treatment, cells were lysed in 50 µL of Lysis Buffer for 10 minutes at RT with shaking (350 rpm). STAT1 Phospho (Tyr701) levels were evaluated by AlphaLISA SureFire Ultra. For the detection step, 10 µL of cell lysate (approximately 8,000 cells) were transferred into a 384-well white OptiPlate, followed by 5 µL of Acceptor mix and incubated for 1 hour at RT. Finally, 5 µL of Donor mix was then added to each well and incubated for 1 hour at RT in the dark.
As expected, IFN type I and II were the main activators of STAT1 phosphorylation in primary macrophages.
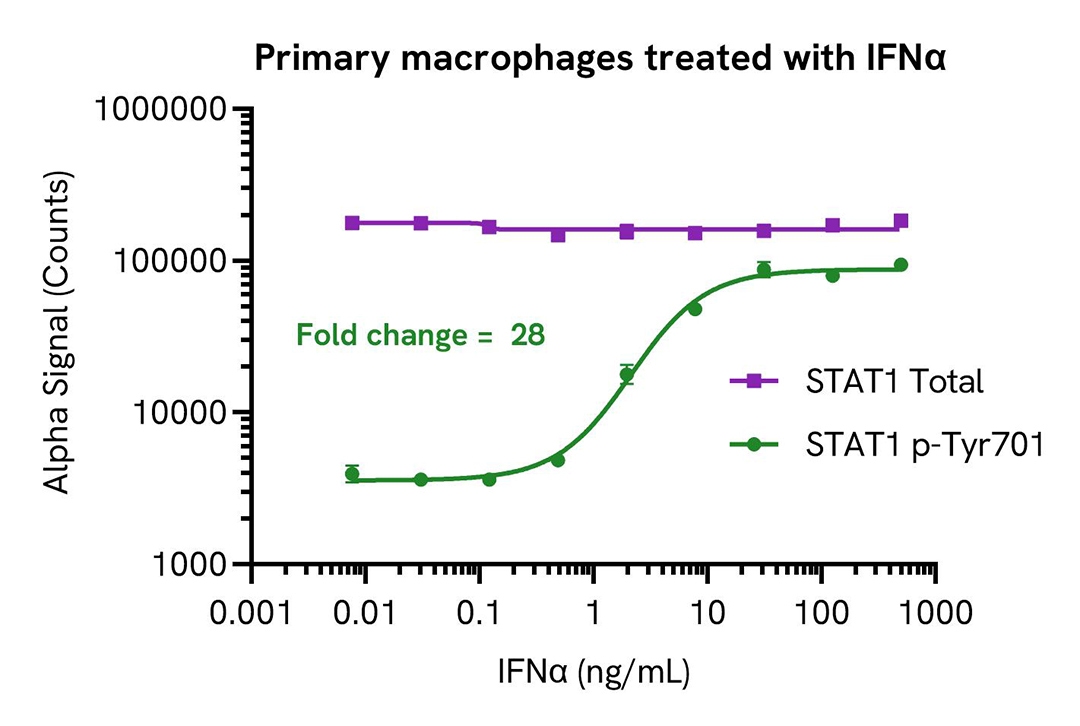
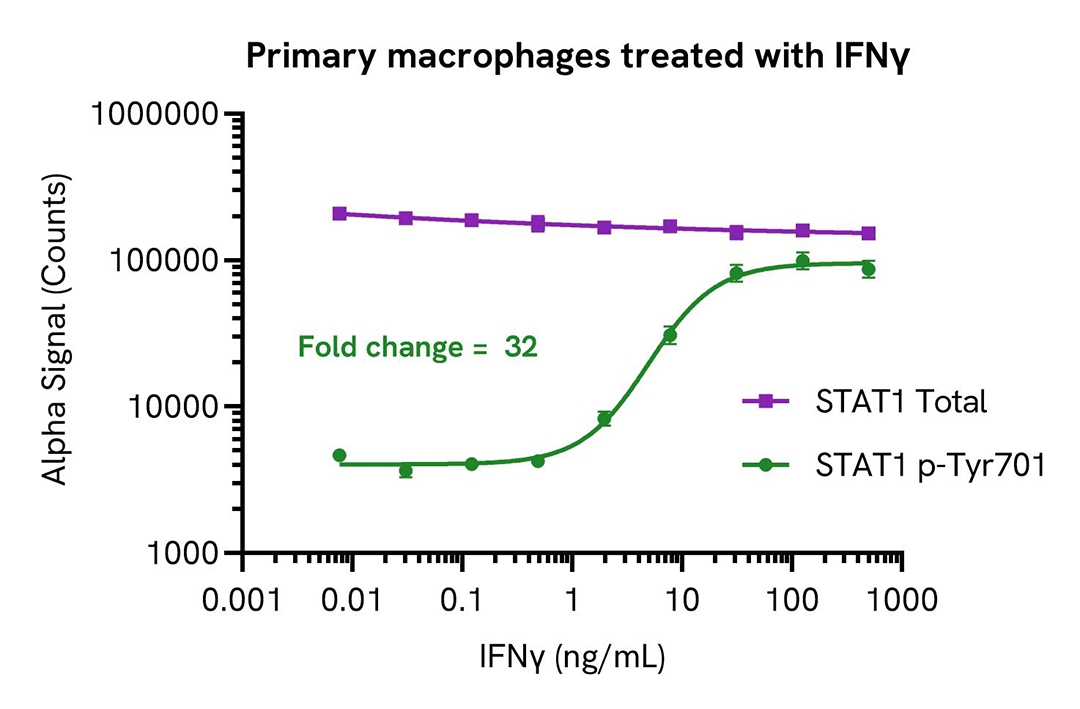
IFNα and IFNγ induce STAT1 phosphorylation in a dose-dependent manner
PBMCs were isolated from healthy donors and cultured for 6 days in complete DMEM containing 20 ng/mL M-CSF to differentiate them into macrophages. Macrophages were seeded in a 96-well plate (40,000 cells/well) in complete DMEM, and incubated overnight at 37°C, 5% CO2. Cells were starved for 2 hours and then treated with IFNα or IFNγ for 20 minutes.
After treatment, cells were lysed in 150 µL of Lysis Buffer for 10 minutes at RT with shaking (350 rpm). STAT1 Phospho (Tyr701) and Total levels were evaluated using respective AlphaLISA SureFire Ultra assays. For the detection step, 10 µL of cell lysate (approximately 2,600 cells) were transferred into a 384-well white OptiPlate, followed by 5 µL of Acceptor mix and incubated for 1 hour at RT. Finally, 5 µL of Donor mix was then added to each well and incubated for 1 hour at RT in the dark.
As expected, IFNα and IFNγ triggered a dose-dependent increase in the levels of Phospho STAT1 (Tyr701) while Total STAT1 levels remained unchanged.
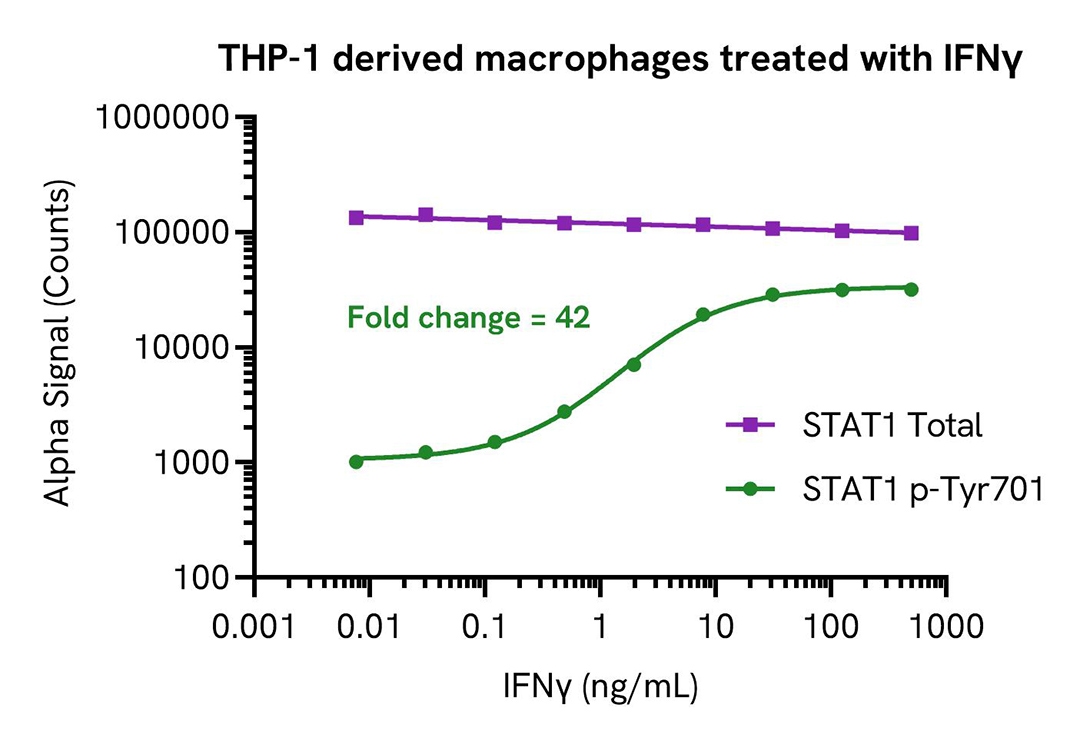
THP-1 cells were seeded in a 96-well plate (100,000 cells/well) in complete medium containing 100 nM of PMA for 24 hours at 37°C, 5% CO2. The THP-1 derived macrophages were starved for 2 hours in HBSS + 0.1% BSA and then treated with increasing concentrations of IFNγ for 20 minutes.
After treatment, the cells were lysed with 60 µL of Lysis Buffer for 10 minutes at RT with shaking (350 rpm). STAT1 Phospho (Tyr701) and Total levels were evaluated using respective AlphaLISA SureFire Ultra assays. For the detection step, 10 µL of cell lysate (approximately 16,000 cells) were transferred into a 384-well white OptiPlate, followed by 5 µL of Acceptor mix and incubated for 1 hour at RT. Finally, 5 µL of Donor mix was then added to each well and incubated for 1 hour at RT in the dark. The plate was read on an Envision using standard AlphaLISA settings.
As expected, IFNγ triggered a dose-dependent increase in the levels of Phospho STAT1 (Tyr701) while Total STAT1 levels remained unchanged.
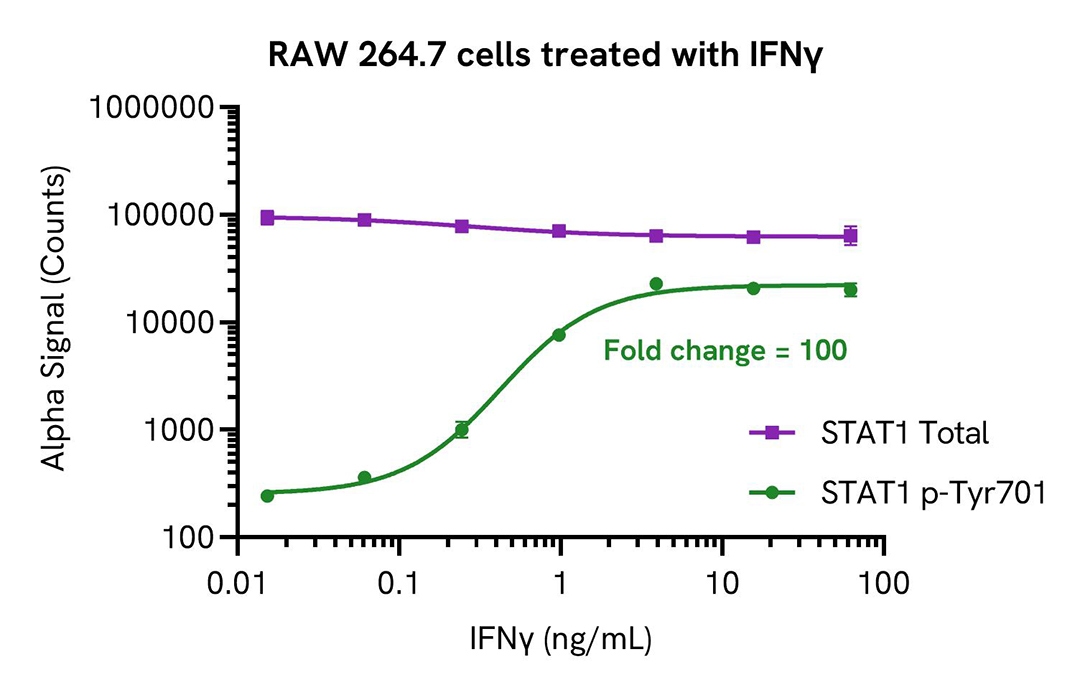
RAW 264.7 cells were seeded in a 96-well plate (40,000 cells/well) in complete medium and incubated overnight at 37°C, 5% CO2. The cells were treated with increasing concentrations of mouse IFNγ for 20 minutes.
After treatment, the cells were lysed with 100 µL of Lysis Buffer for 10 minutes at RT with shaking (350 rpm). STAT1 Phospho (Tyr701) and Total levels were evaluated using respective AlphaLISA SureFire Ultra assays. For the detection step, 10 µL of cell lysate (approximately 4,000 cells) were transferred into a 384-well white OptiPlate, followed by 5 µL of Acceptor mix and incubated for 1 hour at RT. Finally, 5 µL of Donor mix was then added to each well and incubated for 1 hour at RT in the dark. The plate was read on an Envision using standard AlphaLISA settings.
As expected, IFNγ triggered a dose-dependent increase in the levels of Phospho STAT1 (Tyr701) while Total STAT1 levels remained unchanged.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Automation Compatible |
Yes
|
| Brand |
AlphaLISA SureFire Ultra
|
| Cellular or Signaling Pathway |
JAK/STAT
|
| Detection Modality |
Alpha
|
| Lysis Buffer Compatibility |
Lysis Buffer
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
10 µL
|
| Shipping Conditions |
Shipped in Blue Ice
|
| Target |
STAT1
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
Alpha
|
| Unit Size |
500 assay points
|
Video gallery









Citations
Resources
Are you looking for resources, click on the resource type to explore further.
This guide outlines further possible optimization of cellular and immunoassay parameters to ensure the best possible results are...
The definitive guide for setting up a successful AlphaLISA SureFire Ultra assay
Several biological processes are regulated by...
Discover Alpha SureFire® Ultra™ assays, the no-wash cellular kinase assays leveraging Revvity's exclusive bead-based technology...
Atherosclerosis pathogenesis, cellular actors, and pathways
Atherosclerosis is a common condition in which arteries harden and...
The measurement of protein phosphorylation is a useful tool for measuring the modulation of receptor activation by both antibodies...
An in-depth review of molecular and cellular pathways
The maintenance of proteostasis, the biological mechanisms that control the...


Loading...
How can we help you?
We are here to answer your questions.