CHOSOURCE GS KO cell line
Robustness, reliability, and proven track-records to advance biotherapeutic programs
The CHOSOURCE™ Glutamine Synthetase (GS) knockout (KO) cell line is a well-established, cGMP-manufactured CHO-K1-derived suspension cell line, specifically engineered by knocking-out the glutamine synthetase gene to provide a robust industry standard selection system. The GS KO selection system facilitates the selection of high expressing clones during cell line development, accelerating cell line development timelines with high efficiency cell line screening.
Since its introduction in 2016, over 100 licensees have adopted this cell line globally and it has supported over 100 clinical trial authorizations (IND, CTA, CTN). To make regulatory submissions even easier, we have made a drug master file (DMF) submission.
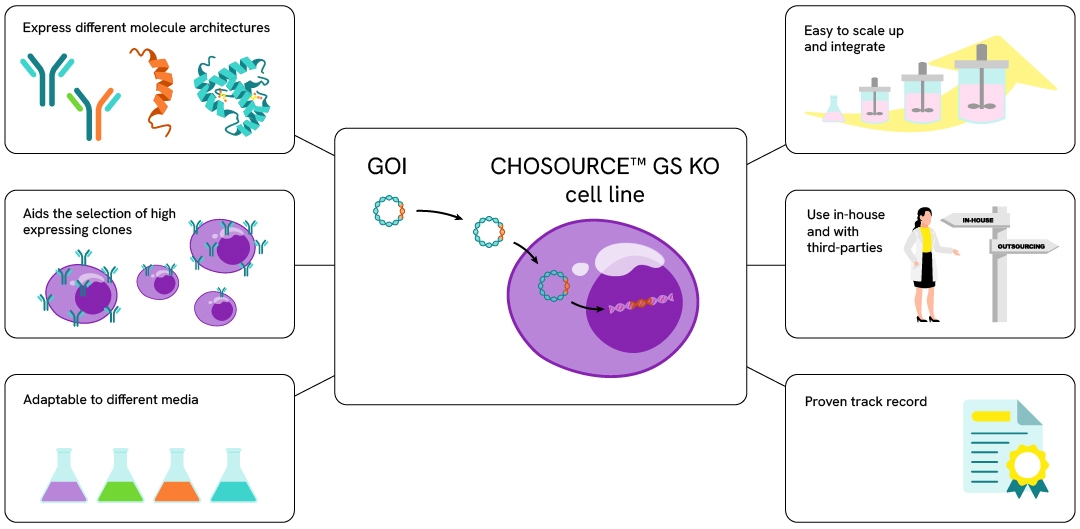
Why choose CHOSOURCE expression platform?
Industry standard
- Easy-to-use CHOSOURCE GS knockout cells have been widely adopted by pharmaceutical companies for bioproduction/manufacturing
- Glutamine Synthetase (GS) selection is the gold standard for selection system and is widely recognized by regulators as suitable for manufacture of biologics
Track record and regulatory compliance
- Utilized by eight of the highest revenue producing pharma companies for their therapeutic development programs
- Fully documented cell line history pack and Drug Master File (DMF) logged with the US FDA
- cGMP cell banks
Truly adaptable platform
- Validated with different feeds, media, processes, scale, molecules (from mAbs to novel scaffolds) and vector technologies
- No restrictions from third parties or media
Dedicated team
- Accessible sales, licensing, and scientific support teams to assist you through the various stages of licensing and setup
- Comprehensive protocols and post-sales scientific support with extensive expertise using the platform to drive advancements in your pipeline
The CHOSOURCE GS KO cell line is available under a non-royalty-bearing commercial license with flexible terms tailored to meet your specific needs.
The CHOSURCE Platform is available for research, clinical, diagnostic, and commercialization applications, including services, under specific licenses from Revvity.
Featured resources





























