Scientists investigating the internalization behavior of 60 GPCRs (G protein-coupled receptors) have found that only about one-third rely entirely on β-arrestins for endocytosis.1 The remainder were either partially β-arrestin dependent or entirely independent, pointing to alternative pathways that have been largely overlooked.
Notably, the study reveals that the GLP-1 receptor (GLP-1R), which is the target of popular weight-loss drugs such as semaglutide (Ozempic), primarily internalizes without β-arrestins but can also use β-arrestin-dependent mechanisms when needed. This surprising finding challenges the long-held assumption that β-arrestins are universally required for GPCR internalization, opening new research directions for receptor signaling and drug development.
Why is GPCR internalization important?
GPCRs are the largest receptor family in the human genome, mediating numerous intracellular signaling pathways in response to diverse extracellular stimuli. After activation by an agonist, these receptors are typically removed from the cell surface by endocytosis. This process prevents overstimulation and allows certain receptors to signal from intracellular compartments.
Traditionally, β-arrestin 1 (βarr1) and β-arrestin 2 (βarr2) were thought to drive this process by binding to activated receptors and recruiting adaptor proteins such as AP2 and clathrin to assemble the machinery required for internalization (Figure 1). However, the relative importance of β-arrestins across the vast GPCR family have remained unclear.

Figure 1: The classical β-arrestin-dependent GPCR internalization pathway. Following ligand activation (1), GPCRs recruit β-arrestins, which in turn recruit AP2 adaptor proteins to initiate clathrin-mediated endocytosis (2). The internalized receptor (3) can then be recycled or degraded.
To address this knowledge gap, Liu, Xue, and their team developed a series of FRET-based assays to examine the internalization behavior of 60 GPCRs expressed in βarr1/βarr2 double knock-out HEK293 cells.
Key study findings
As shown in Figure 2, the receptors fell into four distinct categories based on their internalization behavior:
- Totally β-arrestin-dependent GPCRs: Around one-third of the receptors showed no internalization without β-arrestins. Among these, 17 were rescued by βarr1 overexpression and 19 by βarr2.
- β-arrestin-independent GPCRs. A minority internalized completely independently, with βarr1 or βarr2 overexpression having no effect.
- Partially β-arrestin dependent GPCRs. These receptors internalized in the absence of β-arrestins but showed enhanced internalization when β-arrestins were present.
- No internalization. A small subset showed virtually no internalization under any condition, even when β-arrestins were reintroduced.

Figure 2: Classification of the 60 GPCRs depending on the effect of βarr1 or βarr2 on the agonist-induced internalization.
Interestingly, most of the GPCRs studied demonstrated constitutive internalization that did not require β-arrestins, suggesting a shared baseline mechanism that operates independently of the classical β-arrestin pathway.
A focus on GLP-1R
Detailed analysis of GLP-1R revealed that the receptor internalizes mainly through a β-arrestin-independent mechanism involving direct interaction between the receptor and the clathrin adaptor AP2. However, when this AP2-dependent pathway is disrupted, GLP-1R internalization becomes fully β-arrestin-dependent. This demonstrates how some GPCRs use multiple internalization mechanisms, adapting to cellular conditions.
How Revvity's tools supported this research
Several Revvity technologies supported this research:
β-arrestin detection kits: Revvity's HTRF™ human and mouse total β-arrestin 1 and β-arrestin 2 detection kits (#64BAR1TPEB and #64BAR2TPEB) were used to quantify the levels of β-arrestin in cells, clarifying their relative contributions.

Figure 3: HTRF Total β-arrestin 1 and Total β-arrestin 2 quantification assay principle
DERET internalization assay: The diffusion-enhanced resonance energy transfer (DERET) internalization assay was performed to measure GPCR internalization in real time. GPCRs were expressed with N-terminal SNAP/CLIP tags and labeled using Revvity's Tag-lite SNAP/CLIP labeling medium (#LABMED) and Tag-lite SNAP-Lumi4-Tb labeling reagent (#SSNPTBD). When agonist and fluorescein are added to cells, the Lumi4-Tb on GPCRs at the cell surface transfers energy to the fluorescein in close proximity, triggering fluorescence around 520 nm. As receptors internalize, the extracellular Lumi4-Tb decreases, leading to a proportional drop in fluorescein emission.

Figure 4: Tag-lite GPCR internalization assay principle
GPCR surface expression quantification: To quantify relative receptor levels on the cell surface, SNAP-tagged GPCRs were labeled using Revvity's Tag-lite SNAP/CLIP labeling medium (#LABMED) and Tag-lite SNAP-Lumi4-Tb labeling reagent (#SSNPTBD). Unlike the DERET assay, this method directly measures Lumi4-Tb emission without energy transfer to an acceptor. Lumi4-Tb was excited by a laser at 337 nm, and donor emission was recorded at 620 nm. This approach provides relative quantification, enabling comparison of expression levels across different cell types and receptors.
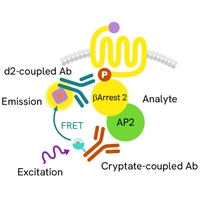
βarr2 and AP2 interaction assays: The team also examined how βarr2 interacts with AP2, a key step in clathrin-mediated endocytosis. Revvity's HTRF β-Arrestin 2 Recruitment kit (#62BDBAR2PEB) allowed the study of these interactions using FRET-based techniques. This assay employs an antibody pair directed against βarr2 and AP2, thereby facilitating detection of their interaction as a readout of βarr2-dependent internalization.

Figure 5: HTRF β-arrestin 2 recruitment assay principle
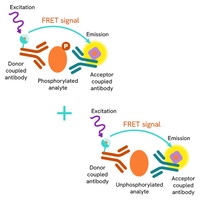
βarr2 and AP2 recruitment assays: To study how receptors recruit βarr2 and AP2 upon activation, the researchers used GPCRs fused to EGFP on their intracellular C-terminus. Cells were incubated with Revvity's terbium-labeled anti-βarr2 or anti-AP2 antibodies, which act as FRET donors while EGFP serves as the acceptor. When agonist stimulation brings βarr2 or AP2 into proximity with the tagged receptor, a TR-FRET signal is generated, enabling detection of endogenous β-arrestin and AP2 recruitment.

Figure 6: HTRF β-arrestin 2 or AP2 recruitment assay principle
Implications for drug development
Understanding the mechanisms of GPCR internalization is critical for drug discovery, as it affects how long receptors remain active and how they signal. This study challenges the traditional view that β-arrestins are universally required for GPCR endocytosis, revealing that some receptors rely heavily on β-arrestins, others bypassing them entirely, and many use both pathways depending on conditions. These findings suggest that drugs targeting the same receptor might work differently depending on which internalization pathway they engage.
To explore how Revvity can support your GPCR research, visit our GPCR reagents page.
Featured products

Reference
- Liu J, Xue L, Ravier MA, Eshak F, Acher FC, Goupil-Lamy A, Cimadevila M, Drube J, Hoffmann C, Inoue A, Trinquet E, Dupuis E, Prézeau L, Pin J-P and Rondard P. (2025) Multi-faceted roles of β-arrestins in G protein-coupled receptor endocytosis. Nat Comm in press. doi.org/10.1038/s41467-025-67156-y
For research use only. Not for use in diagnostic procedures.