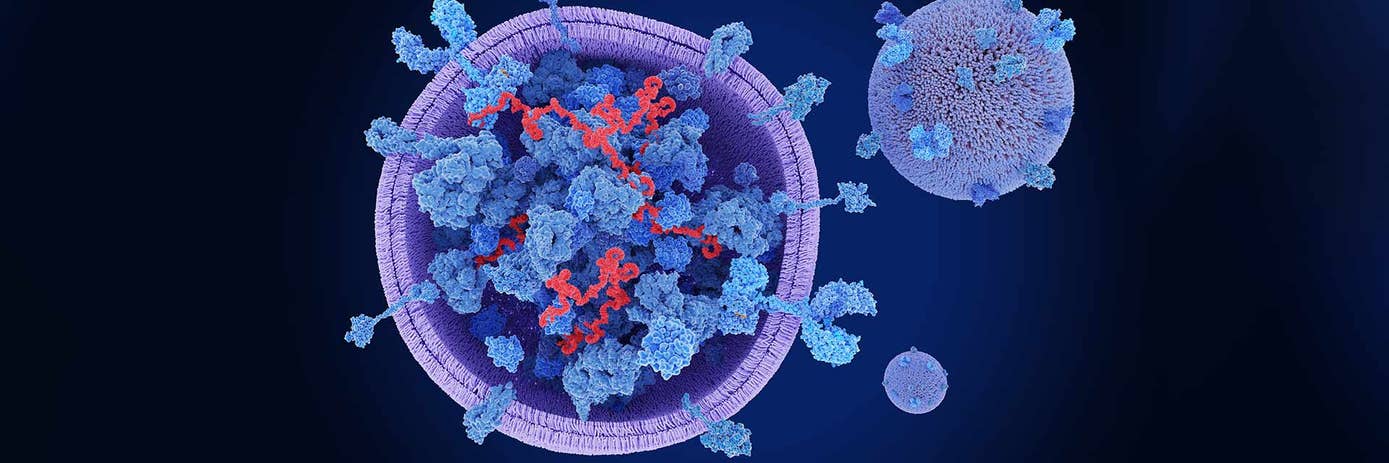
As demand grows for more precise non-invasive biomarkers, extracellular vesicles (EVs) have emerged as promising tools for disease research. Secreted by virtually all cell types, including cancer cells, these nano-sized membrane-bound particles circulate abundantly in biological fluids like blood, urine, and saliva, making them ideal candidates for non-invasive liquid biopsy applications.
What makes EVs particularly valuable is their diverse cargo of proteins, lipids, and nucleic acids, all encapsulated within a phospholipid bilayer. This “molecular fingerprint” reflects the state of their parent cells, offering insights into cellular function and disease processes without the need for invasive tissue sampling.
In cancer research, EVs have proven especially promising. Tumor-derived vesicles carry cancer-specific molecules that can shed light on tumor heterogeneity, metastatic potential, and mechanisms of therapeutic resistance. At the same time, their role in cell-to-cell communication lends itself to tracking disease progression and assessing treatment response.
Evolving isolation and analysis technologies
Making the most of the information contained within EVs requires effective ways to isolate and characterize these vesicles. While traditional isolation techniques like ultracentrifugation remain the gold standard, they come with significant limitations: the workflow is relatively slow, labor intensive, and typically requires large sample volumes, making them suboptimal for high-throughput or multi-omics workflows.
Recent advances are addressing these limitations with next-generation isolation and analysis platforms designed for higher throughput, greater reproducibility, and compatibility with small sample volumes.
Among these emerging approaches, two studies stand out:
- Ku et al. developed an automated microfluidic “acoustic trap” platform capable of enriching EVs from small samples of urine. When combined with Revvity’s NEXTFLEX™ Small RNA-Seq kit, this approach enables high-throughput miRNA profiling from minimal sample volumes, outperforming alternative methods.1
- In a second study, Sun and Meckes developed a method for detecting EV surface proteins using BioLegend’s TotalSeq™-A antibodies conjugated with DNA oligonucleotides. This technique allows multiplex protein profiling from as little as 10 µL of human serum in under five minutes.2
These technical advances are breaking down traditional barriers to EV analysis, supporting scalable biomarker discovery for personalized medicine applications.
Pioneering solutions for next-generation biomarkers
As methods continue to improve, the ability to monitor changes in EV content over time could revolutionize the way we diagnose and monitor a wide range of diseases—not only in oncology but also for neurodegenerative and cardiovascular conditions. These tools enable longitudinal, in depth insights into disease biology that were previously difficult to achieve.
Our latest literature review provides an in-depth analysis of the technical workflows and performance data behind these novel approaches. By combining acoustic trapping with Revvity’s RNA-Seq technology and leveraging antibody-oligo conjugates for multiplexed surface protein detection, researchers can now overcome longstanding technical challenges that have limited progress in the field. Download the literature review to discover how these innovative techniques are accelerating biomarker discovery by extracting more data and insights while lowering sample requirements.
For research use only. Not for use in diagnostic procedures.
References
- Ku A, Ravi N, Yang M, Evander M, Laurell T, Lilja H, et al. A urinary extracellular vesicle microRNA biomarker discovery pipeline; from automated extracellular vesicle enrichment by acoustic trapping to microRNA sequencing. PLoS ONE. 2019;14(5):e0217507. https://doi.org/10.1371/journal.pone.0217507
- Sun L, Meckes DG. Multiplex protein profiling method for extracellular vesicle protein detection. Sci Rep. 2021;11:12477. https://doi.org/10.1038/s41598-021-92012-6