Stepping into a laboratory of a leading medical diagnostic provider, it is a hustle of activity along the long white corridor. Laboratory staff glided swiftly from room to room. Within one room, a nonchalant scientist sorted through a multitude of tube types containing various body fluids into different racks, the colorful mosaic of rubber stoppers building up in a pile in front of her. Clinical laboratories like the one above are facing what may seem like an insurmountable challenge. The number of available genetic tests has been growing at an unprecedented rate, from 1000 in 2012 to over 75 000 in 2022 worldwide1. Fuelled by technological advancements, growing consumer awareness, and the push for precision medicine, medical applications of genetic testing have multiplied especially in the fields of oncology2,3, reproductive health4, and pharmacogenomics5. Laboratories today are under enormous pressure to scale up their operations and quicken their turnaround times. All while maintaining a high level of traceability and quality control that fulfils regulatory requirements. Cue the use of automation as laboratories become increasingly dependent on robots to pipette the reagents needed to lyse cells, extract nucleic acids, and analyse the all-informing genetic information within. But as labs strive to automate their nucleic acid purification and scale up their operations, challenges start to arise. Here, we cover the common challenges laboratory managers face and ways to manage, when transitioning from manual to automated sample preparation, or when scaling up existing operations.
Challenges faced by clinical laboratories implementing or scaling up automated nucleic acid purification:
-
High upfront costs with long-term return of investment
Equipment, reagents and service contracts add up and costs have to be justified to funding bodies or management. Despite the high upfront investment, laboratory managers should understand the long-term return of investment automation can bring.

A laboratory offering testing for human and veterinary samples was dealing with 12 different sample types, performing 5 extractions with 23 variations of sample pre-treatment. After implementing the chemagic extraction platform from Revvity, they were able to consolidate workflows to 1 extraction with 2 pre-treatment variations resulting in:
- 30% decreased cost/reaction (~USD$100 000 annual savings)
- Increased throughput from 400 samples/day with 2 technicians to 700 samples/day with 1.2 technicians
- Reduced repeat rates from 3.4% to 0.7%
This involves not just potential cost-savings from reduced labor costs, increased throughput and decreased repeat rates, but also intangible benefits of improved reproducibility and standardization, enhanced data management and traceability, as well as staff satisfaction and retention. Notably, working with a single supplier that caters to full workflows can lead to significant cost savings from attractive product bundles. Furthermore, an automated nucleic acid purification system where both reagents and instrumentation come from the same supplier, can facilitate more efficient implementation.
Time is saved from the reduced administrative overhead of dealing with multiple vendors, performance is highly optimized and technical service and support can be provided more efficiently, resulting in higher customer satisfaction.
-
Integrating with existing workflows
Adapting current protocols to automation, ensuring compatibility with downstream assays and managing the transition period are crucial factors to consider for laboratories automating their sample preparation. It is important to choose a flexible nucleic acid extraction system that can handle diverse workflows while achieving a quality of nucleic acids compatible for downstream assays. Thought should also be given to future scaling and how easily this can be achieved. Ask yourself these questions when choosing a system:
Can the automated system handle all current workflows and sample volumes at required throughputs? Is the quality and yield of nucleic acids suitable for testing assays or future test assays? How easy is it to change the system to deal with a higher or lower throughputs when needed?
Once a system has been chosen, a comprehensive plan is needed to verify the new method with acceptance criteria established for successful integration. Standard operating procedures would need to be set up that define how to operate, troubleshoot and maintain the system. A transition timeline with implementation phases and goals that are widely communicated to all stakeholders is also key to get everyone aligned and on board.
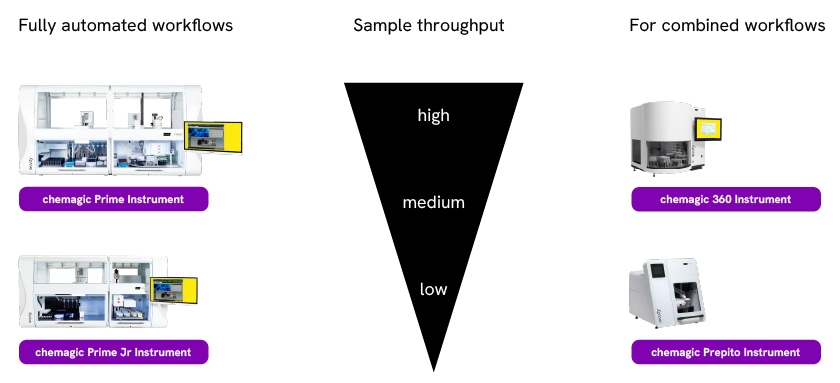
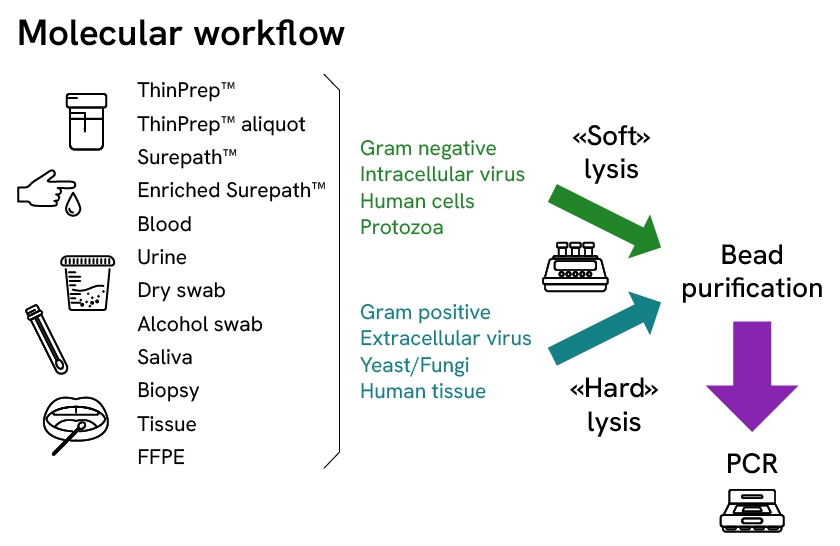
Extraction performance can vary when scaling from manual column-based approaches to magnetic bead- based approaches. Variations in nucleic acid quality can occur even between different bead-based automated platforms. All chemagic instruments, from the low throughput chemagic Prepito (12 samples/run) to the high throughput chemagic Prime (192 samples/run), rely on the same separation technology with similar kit chemistries optimized for various targets. This minimizes performance discrepancies when scaling throughputs and avoids excessive validation efforts. In addition, the chemagic systems have a uniquely wide sample volume range (50 µl to 18 ml) and capability to extract high molecular weight (HMW) DNA with a ~1 h processing time, enabling its application to diverse workflows even with demanding downstream applications.
-
Data management and integration
Automation greatly helps improve data integrity and accuracy and provides a detailed audit trail for full sample traceability throughout the workflow. Laboratory information management systems (LIMS) play a crucial role in in managing the output from these automated systems. Laboratory managers must ensure new equipment are compatible with their LIMS and work with data formats that allow a seamless transfer of information. Choosing a system with an open application support interface (API) can be greatly beneficial to avoid manual data entry, workflow disruptions, data silos and reduced operational flexibility and compliance issues. Special attention should be given as well to data standardization and mapping. Training needs should be adequately met should the new equipment require any updated LIMS interfaces. Be clear with communicating your needs to your vendor and use the services provided to ensure proper integration and validation of the system.
-
Staff training or acceptance
Although automation helps relieve the workload of laboratory staff, change always involves some resistance and there is a learning curve to be conquered. Communication is vital to get everyone on board, engaged and invested. Sufficient time should be dedicated for training, and support from the vendor should be provided pre- and post-installation. Systems can vary in user-friendliness and for complex liquid handling systems, a certain level of programming knowledge is beneficial to avoid dependence on vendors that charge for developing new protocols. With chemagic systems, protocols come ready-to-use and support is provided when kit or protocol customizations are needed.
-
Space and infrastructure
Automated nucleic acid purification systems come in all shapes and sizes so a thorough site check is needed to ensure proper installation. Space and weight limits, electrical power requirements, environmental conditions such as temperature and humidity, and IT infrastructure for data management should be considered.
It is also key to go through what is needed for the entire unpacking, unloading and transportation process. Doorways and corridors should be measured and steps avoided for the transport of large and heavy instruments.
Modular automation solutions that can be taken apart when moved or offer the ability to scale in future provide greater flexibility. Instruments that allow for vertical integration can maximize floor space efficiency.
Note also the space and infrastructure needed to store consumables as automation can require a significant inventory of pipette tips, reagents, and other plastic consumables. Take note as well as of waste handling processes and if this may require any adaptations to existing systems.
Conclusion
The journey to mastering automation is a long but rewarding one. It transforms the way laboratories work and positively impacts testing turnaround times, the quality of data output and most of all, case and lab safety.
Learn more about Revvity’s automated nucleic acid purification solutions.
For research use only. Not for use in diagnostic procedures.
References:
- Halbisen AL, Lu CY. Trends in Availability of Genetic Tests in the United States, 2012-2022. J Pers Med. 2023 Apr 6;13(4):638. doi: 10.3390/jpm13040638. PMID: 37109024; PMCID: PMC10142561.
- Frank, M. S., Andersen, C. S. A., Ahlborn, L. B., Pallisgaard, N., Bodtger, U., & Gehl, J. Circulating Tumor DNA Monitoring Reveals Molecular Progression before Radiologic Progression in a Real-life Cohort of Patients with Advanced Non–small Cell Lung Cancer. Cancer Research Communications, 2022; 2(10), 1174–1187.
- Callesen LB, Hansen TF, Andersen RF, Pallisgaard N, Kramer S, Schlander S, Rafaelsen SR, Boysen AK, Jensen LH, Jakobsen A, Spindler KG. OPTIMISE: Optimisation of treatment selection and follow-up in oligometastatic colorectal cancer - a ctDNA-guided phase II randomised approach. Study protocol. Acta Oncol. 2022 Sep;61(9):1152-1156.
- Balciuniene J, Liu R, Bean L, Guo F, Nallamilli BRR, Guruju N, Chen-Deutsch X, Yousaf R, Fura K, Chin E, Mathur A, Ma Z, Carmichael J, da Silva C, Collins C, Hegde M. At-Risk Genomic Findings for Pediatric-Onset Disorders From Genome Sequencing vs Medically Actionable Gene Panel in Proactive Screening of Newborns and Children. JAMA Netw Open. 2023 Jul 3;6(7):e2326445. doi: 10.1001/jamanetworkopen.2023.26445. PMID: 37523181; PMCID: PMC10391308.
- Larrue, R., Fellah, S., Hennart, B. et al. Integrating rare genetic variants into DPYD pharmacogenetic testing may help preventing fluoropyrimidine-induced toxicity. Pharmacogenomics J 24, 1 (2024). https://doi.org/10.1038/s41397-023-00322-x

































