Viral titer is an essential assay for researchers studying infectious disease, pathogenesis, vaccine development, even cell and gene therapy. The main goal is to determine the appropriate viral concentrations to be utilized in downstream assays such as antibody neutralization, ELISA-based neutralization, antiviral therapeutic discovery, cytopathic effect (CPE), or simply lentiviral transduction for gene and cell therapy.
Specifically for virology research, infectious viral titers are critical for clinical research and testing. The traditional measurement of viral titers is extremely laborious, subjective and the data points are often limited due to manual experimentation.
What is an infectious viral titer assay?
There are multiple methods to measure the amount of virus in the sample, such as Real-Time (RT) PCR, Western Blot, ELISA, and flow cytometry. These methods utilize the amount of viral DNA, RNA, or proteins to quantify the virus. However, these methods do not measure the actual biological activities of the virus. In order to investigate the infectivity of the virus, in vitro assays are required to assess the neutralizing ability of antibody candidates as well as the effectiveness of antiviral small molecule drugs. An infectious viral titer assay is conducted to determine the strength of a virus against the host cells.
How to measure infectious viral titer?
Viral infectivity is a critical characteristic of an infectious virus, which shows the ability of the virus to infect and replicate in host cells. In order to determine viral infectivity, there are two categories of assays that can be performed. In the first category are quantitative assays that include plaque formation assays (PFA) and focus formation assays (FFA), which can quantify viral particles. The second category contains end-point dilution assays that include 50% tissue culture infective dose (TCID50), lethal dose (LD50), and egg infective dose (EID50).
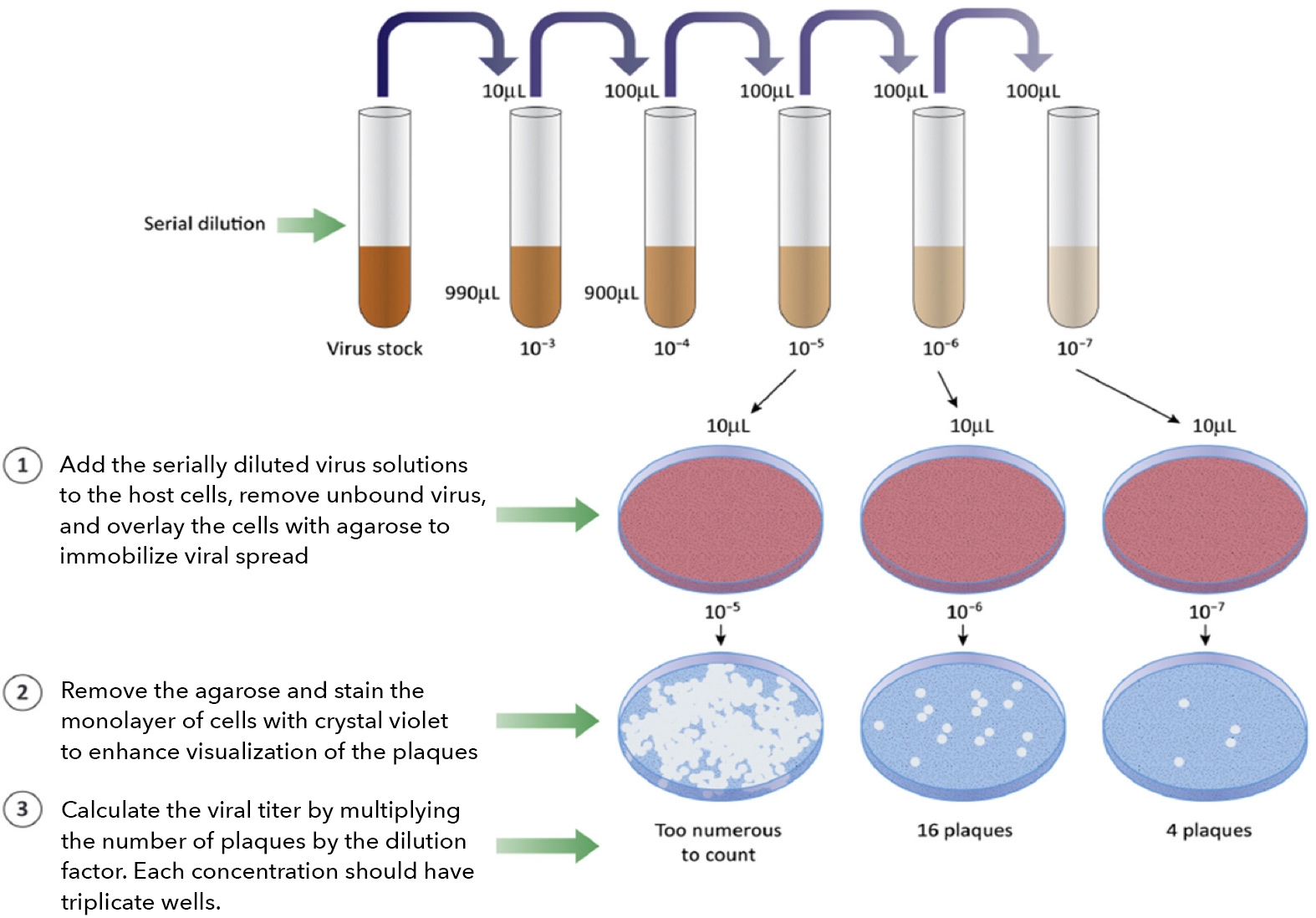
It is common practice to perform a serial dilution of the viral stock, infect host cells, and measure the infectivity and viral titer using a variety of methods. Traditionally, viral titers are quantified by the number of formed plaques, foci, or individually infected cells. The plaque formation assay (PFA) and focus formation assay (FFA) are typically counted with the naked eye or microscopy (light/fluorescence). Individual infected cells can be measured by a conventional flow cytometer.

| Detection method | Description | Existing issues |
|---|---|---|
| Naked Eye (lightbox) | Manual counting of large cytolytic plaques (PRNT) stained with crystal violet in 6- or 12-well plates. | Low-throughput, labor-intensive, time-consuming, operator-variation, requires large volume (6, 12, 24-well plates), no digital records |
| Standard Microscopy | Manual counting of small foci or individual infected cells labeled with horseradish peroxidase in 96-well plates. | Low-throughput, labor-intensive, time-consuming, operator-variation, no digital records |
| Digital Automated Microscopy | Automatic acquisition of fluorescent images of small foci or individual infected cells labeled with fluorescent probes (i.e., immunostaining or fluorescent proteins). Next, use of external software (i.e., ImageJ) to automatically analyze and count the small foci or individual infected cells. | Time-consuming, not streamlined, requires multiple steps |
| Flow Cytometry | Automatic counting and analysis of the population of individual infected cells labeled with fluorescent probes (i.e., immunostaining or fluorescent proteins). | Time-consuming, requires trypsinization of host cells from plates, cannot detect large plaques or foci, must use fluorescent labeling |
What is cytopathic effect (CPE)?
Cytopathic effect (CPE) is the effect of the viral infection on the host cells. The common visual observations of the host cells are swelling or shrinkage, rounding, lysis, clumping, syncytia, and inclusions.
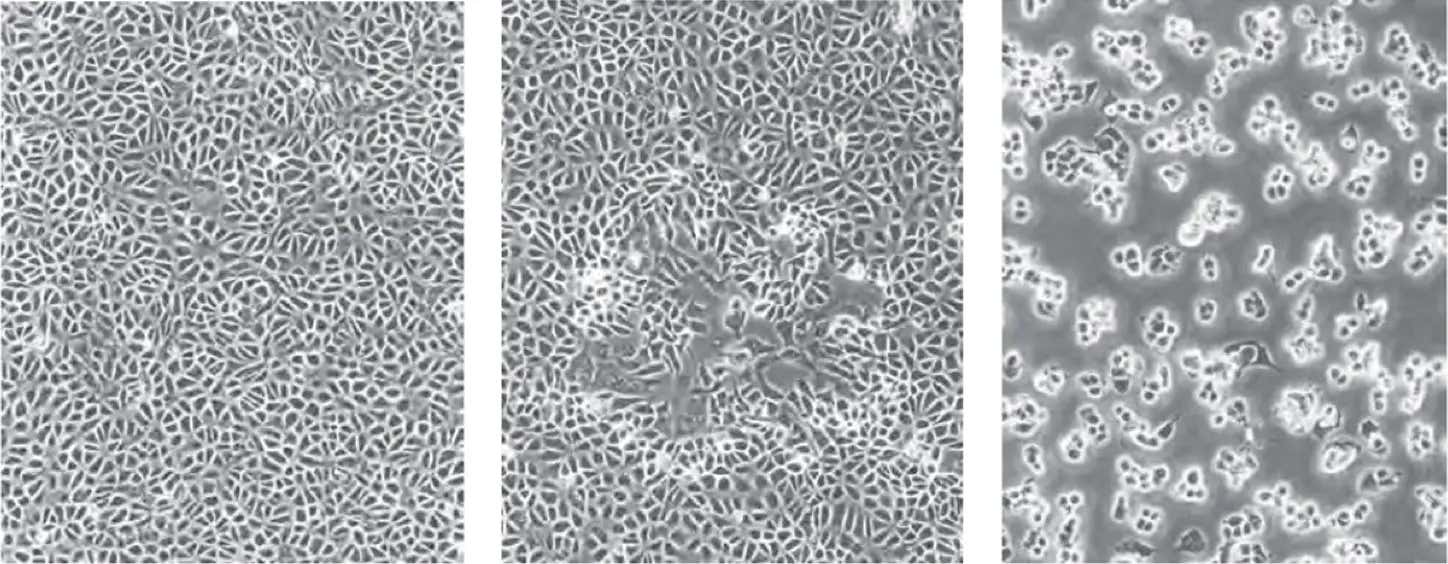
BSC40 cells (left), moi <0.01 pfu/cell 48 hours after infection (center), moi=10 pfu/cell (right)
What is plaque formation assay (PFA)?
When the virus-infected host cells lyse due to the infection, this can generate openings in the monolayer of host cells, forming a plaque. Depending on the type of infectious virus, the formation of the plaques may require 3 – 14 days. In addition, the host cells, media, and pH can affect the size and number of the plaques. This assay requires days to complete, rendering it low-throughput. Manual counting by eyes can be tedious and time-consuming. Operator-to-operator variation has a high impact on the consistency of the results. The time required for plaque formation is long due to the need for plaques to become large enough for manual counting.
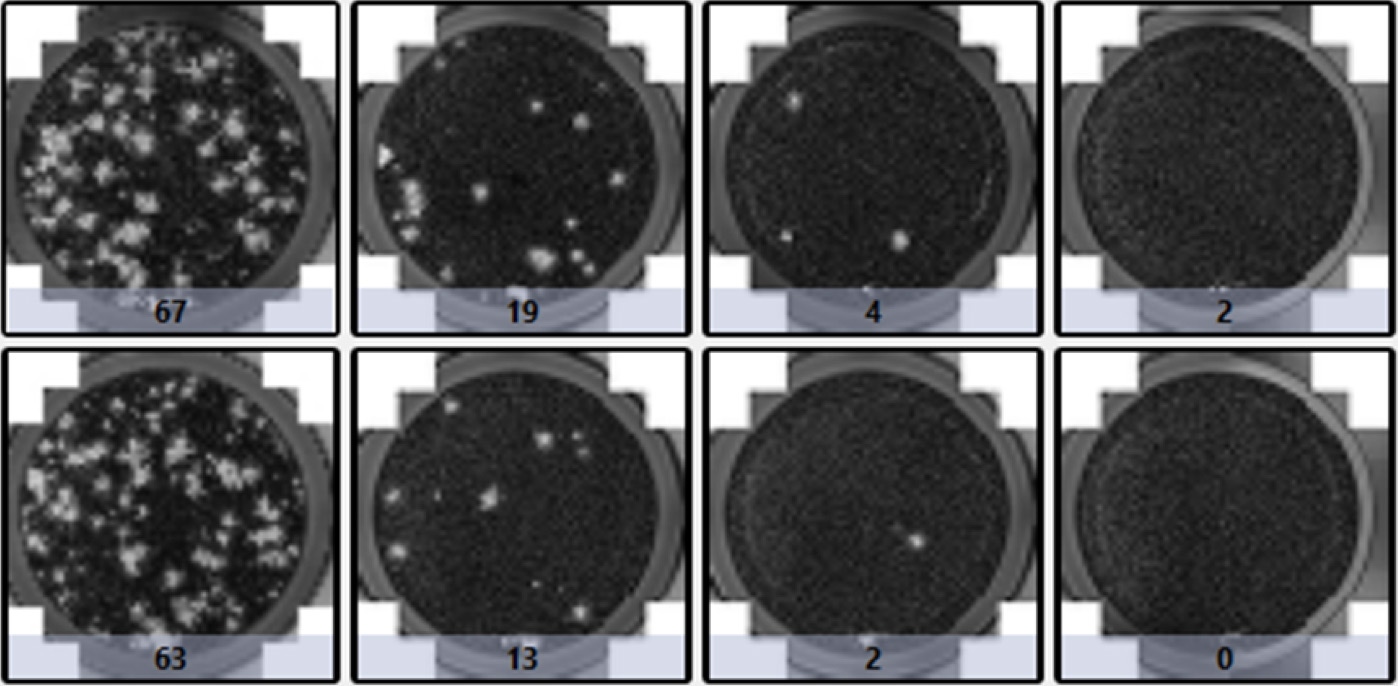
| Plaque count | |||
|---|---|---|---|
| 67 | 19 | 4 | 2 |
| 63 | 13 | 2 | 0 |
| Plaque size (µm2) | |||
| 453017.801 | 478668.211 | 375579.65 | 58492.7559 |
| 481462.961 | 313941.745 | 382699.547 | NaN |
What is focus formation assay (FFA)?
Typically, the focus formation assay is performed when the infectious virus does not lyse the host cells but can still infect the surrounding cells. This generates clusters of infected cells called focus or foci. Foci can be specifically labeled through the use of horseradish peroxidase (coupled with primary anti-viral antibody), fluorescent viral protein markers, or fluorescent virus reporters, which can improve visualization and counting. In addition, unlike plaques, focus formation requires a shorter time to form and to be counted.
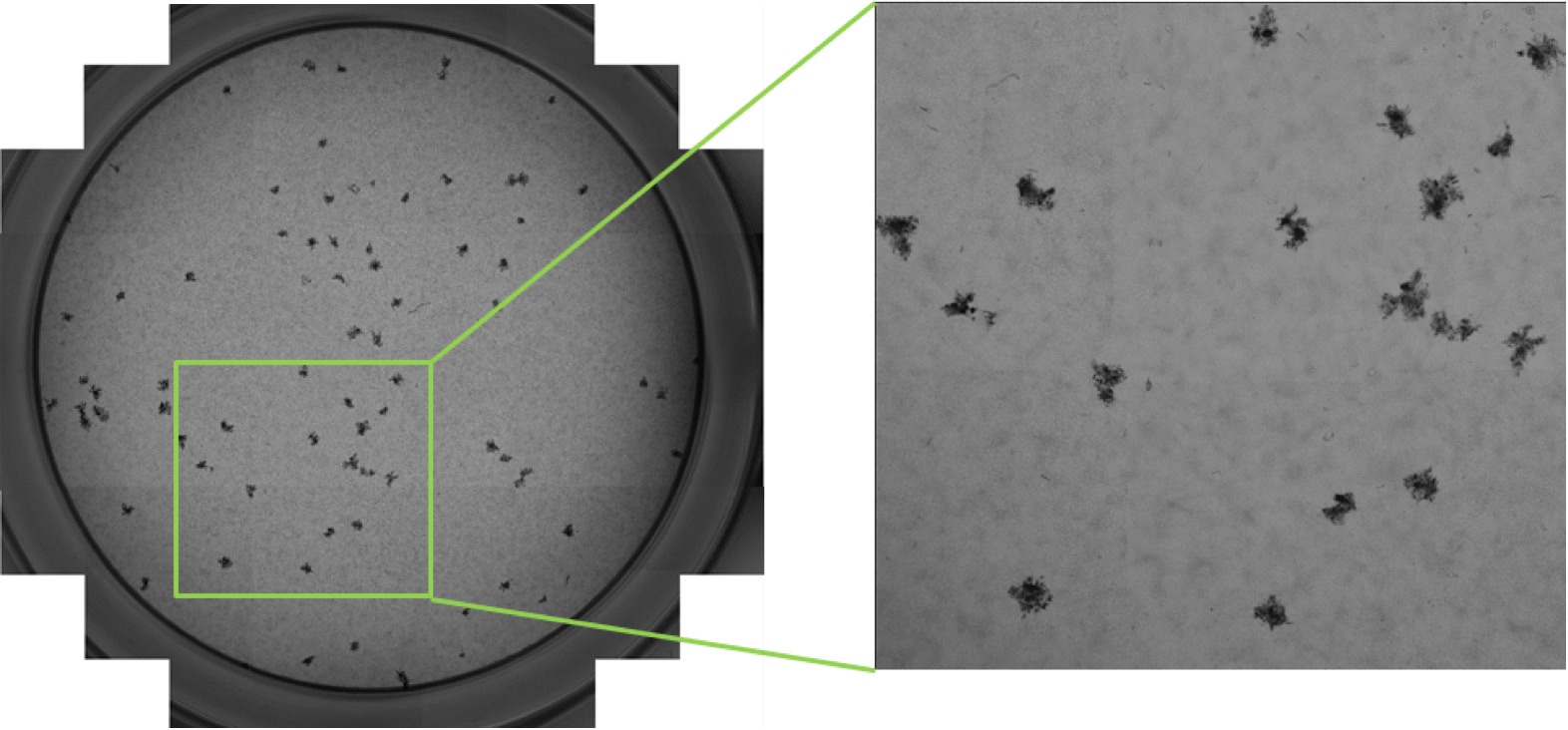
Automated plaque, foci and individual infected cell counting using the Celigo Image Cytometer
The Celigo image cytometer is a high-throughput and high-speed plate imager that can rapidly image the entire microplate in brightfield and fluorescence, to produce counts, morphology, and intensity measurement. In order to characterize the behavior of viral infection, scientists can perform high-throughput automated imaging and analysis directly in plates to directly count infected cells in plates without trypsinization.
- Speed: Less than 10 min/plate with our automated image acquisition and analysis tools
- Innovation: Count every infected and total cells in every well without trypsinization
- Faster results: Earlier plaque detection reduces assay duration
- Single system: Automated brightfield and fluorescence-based counting for focus formation assay
- Convenience: Integratie a plate stacker with up to 50 plates analyzed per day
General viral titer assay protocol for counting horseradish peroxidase or fluorescently labeled individual infected cells
- The host cells are seeded and allowed to adhere to the surface of the 96-well microplates for 24 hours
- Different concentrations or amount of virus particles are added to the plate for measuring the infectivity of the virus
- The infected cells are fixed and labeled with primary anti-viral antibodies and secondary horseradish peroxidase (HRP) or directly conjugated fluorescent antibodies, or fluorescent viral proteins
- The host cell samples are then imaged and analyzed using Celigo image cytometer to count the number of infected cells or % infection
For research use only. Not for use in diagnostic procedures.




























