Drug Development

Transforming Drug Development
The process of drug development comprises all the activities involved in bringing a new drug to market once a lead compound has been identified. Once a single compound is selected, preclinical studies are performed to evaluate whether it is safe, effective at treating the condition it was developed for, and to determine the correct dosage and appropriate administration route.
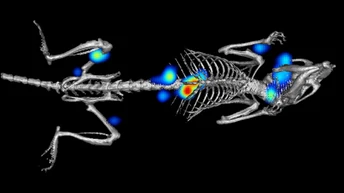
Both in vitro and in vivo models are typically used for evaluating drug candidate safety and efficacy prior to human clinical trials, providing critical information on a compound’s biological effects at this early stage in drug development. Visualizing and evaluating drug effects at the cellular level or in the context of an animal model can provide valuable information on efficacy, biodistribution, toxicity, and safety.
Throughout the drug development process chemistry, manufacturing, and control (CMC) testing is required to ensure the drug product consistently meets pre-determined quality standards. Drug developers must also continuously adhere to current good manufacturing practices (cGMP). Before clinical testing with humans begins, an Investigational New Drug (IND) application is filed with the FDA or relevant regulatory body. If the IND application is approved, then clinical trials can begin.
Efficiency in drug development is critical for commercial success. In every stage of the drug development process, we support our pharmaceutical partners to develop and commercialize new small molecule entities across a wide variety of therapeutic areas at an accelerated pace.
Drug development also produces vast amounts of data at every step of the workflow, all of which requires management and interpretation. We provide comprehensive and innovative line of instrumentation, software, reagents, and consumables, as well as advanced informatics and services that assist our customers to meet compliance requirements throughout the development process.
For research use only. Not for use in diagnostic procedures.
Key benefits:
- End-to-end solutions for the drug development lifecycle.
- Advanced analytical tools for biotherapeutic characterization.
- Support for regulatory compliance and drug safety assessment.
- Integrated platforms for efficient clinical trial management.
Explore our solutions
Questions?
We’re here to help.
Contact us Please note that product labeling (such as kit insert, product label, and kit box) may be different compared to the company branding. Please contact your local representative for further details.